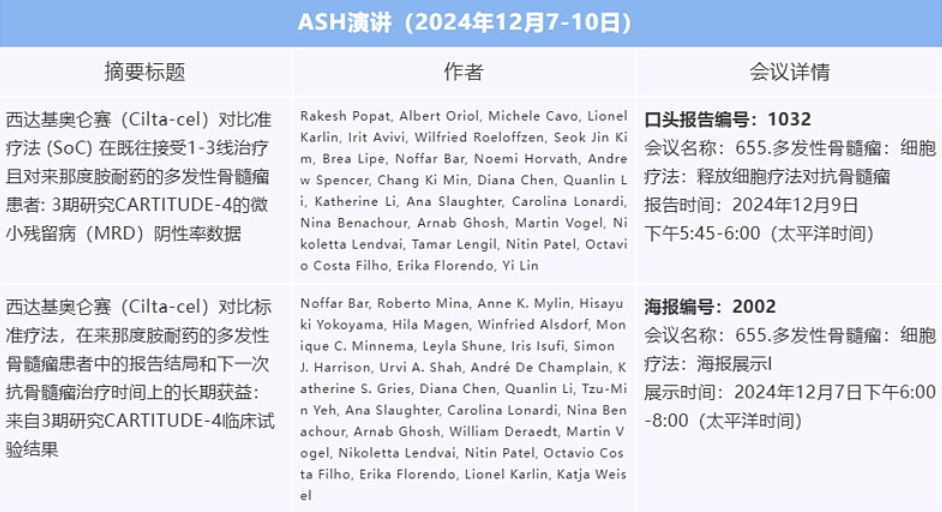
China-based Legend Biotech Corporation (NASDAQ: LEGN) is set to present new data on minimal residual disease (MRD) negativity rates from the Phase III CARTITUDE-4 trial at the 66th Annual American Society of Hematology (ASH) Annual Meeting in San Diego. The data will compare Carvykti (ciltacabtagene autoleucel; cilta-cel) treatment with the standard of care (SoC) in multiple myeloma patients.
Significance of CARTITUDE-4 Trial Data
Previously released data from the CARTITUDE-4 trial indicated that a single infusion of Carvykti significantly prolonged overall survival (OS) in multiple myeloma (MM) patients who had previously received at least first-line treatment, including a proteasome inhibitor and immunomodulatory agent, and experienced relapse or lenalidomide resistance. The risk of patient mortality was reduced by 45% compared with pomalidomide, bortezomib, and dexamethasone (PVd) or daratumumab, pomalidomide, and dexamethasone (DPd).
Upcoming Data Release and Implications
The upcoming data presentation will evaluate the MRD negative rates of the two regimens. The results demonstrate that Carvykti can significantly improve the MRD negative rate and maintain MRD negative status compared to standard treatment. This improvement is expected to enhance the long-term prognosis of MM patients, offering a potentially transformative treatment option.- Flcube.com