China-based Everest Medicines (HKG: 1952) has announced that it has received full approval from the Ministry of Food and Drug Safety (MFDS) in South Korea for its Nefecon (targeted-release budesonide) for the treatment of adults with primary immunoglobulin A nephropathy (IgAN) who have a urine protein excretion of ≥1.0 g/day (or urine protein-to-creatinine ratio ≥0.8 g/g).
Nefecon’s Unique Treatment Profile
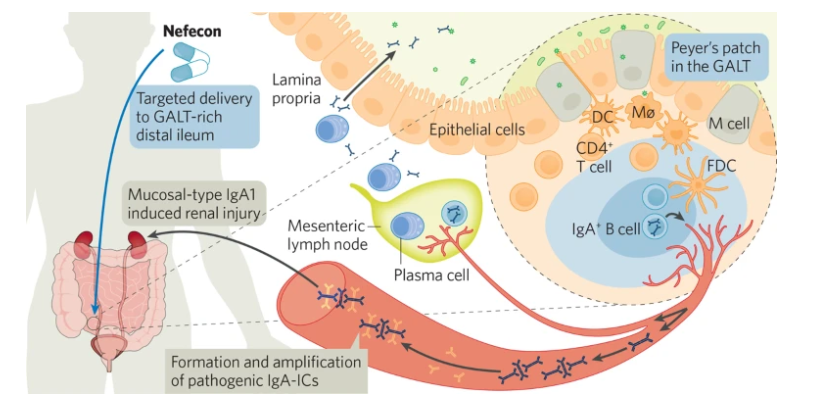
Nefecon is recognized as the world’s only etiological treatment for IgAN. It is a patented oral, delayed-release formulation of budesonide, a corticosteroid with potent glucocorticoid activity and weak mineralocorticoid activity that undergoes substantial first-pass metabolism. This targeted approach to treatment offers a significant advancement in managing IgAN, a disease that affects kidney function.
Everest Medicines’ Expansion and Licensing Agreement
In June 2019, Everest Medicines entered into an exclusive, royalty-bearing license agreement with Calliditas, which granted Everest Medicines exclusive rights to develop and commercialize Nefecon in Mainland China, Hong Kong, Macau, Taiwan, and Singapore. This deal was expanded in March 2022 to include South Korea, further extending the reach of Nefecon in the Asia-Pacific region.-Fineline Info & Tech