China-based Huadong Medicine Co., Ltd (SHE: 000963) has announced receiving marketing approval from the National Medical Products Administration (NMPA) for its drug Arcalyst (rilonacept), indicated for the treatment of cryo-pyrin-associated periodic syndromes (CAPSs), including familial cold autoimmune syndrome (FCAS) and muckle-well syndrome (MWS).
Understanding CAPS and Its Subtypes
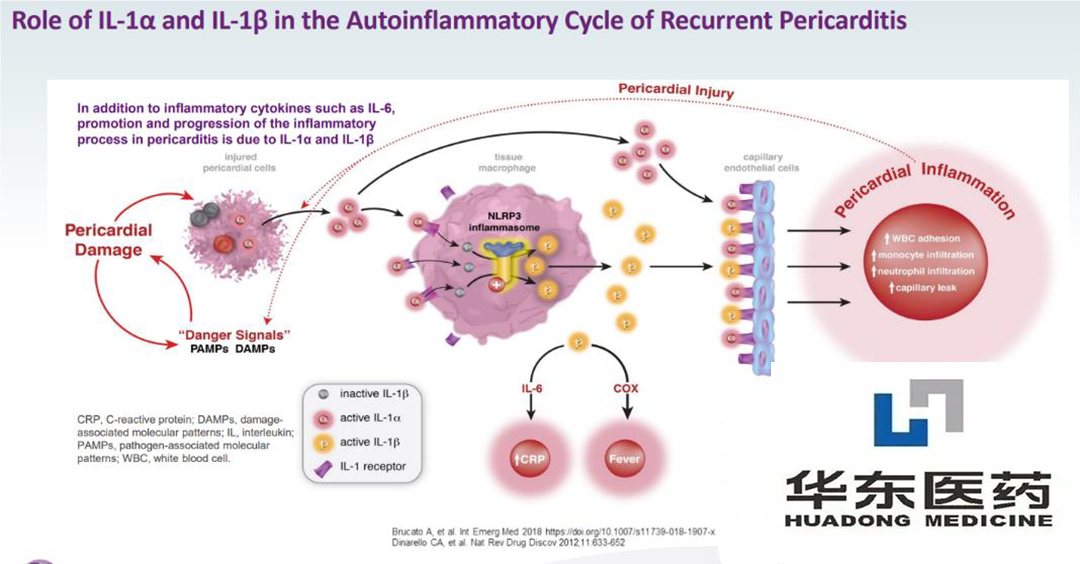
CAPS is a rare autoimmune disease that encompasses three subtypes: FCAS, MWS, and neonatal-onset multisystem inflammatory disease or chronic infantile neuro cutaneous joint syndrome (NOMLD/CINCA). These subtypes share a common clinical manifestation: recurrent multi-system inflammation affecting the skin, muscles, bones, joints, eyes, ears, and central nervous system (CNS).
Rilonacept’s Mechanism of Action and Approval History
Rilonacept is a recombinant dimer fusion protein that blocks interleukin-1 α (IL-1 α) and interleukin-1 β (IL-1 β) signal transduction. Originated by Regeneron Pharmaceuticals Inc., it was approved for marketing in the United States in 2008 and later gained approval to treat IL-1 receptor antagonist deficiency (DIRA) in 2020. Kiniksa Pharmaceuticals Ltd (NASDAQ: KNSA) obtained a license to the drug in 2017, which received breakthrough therapy designation (BTD) for use in recurrent pericarditis (RP) in 2019 in the US. Orphan drug designations (ODDs) were awarded in the US and Europe in 2020 for the treatment of pericarditis and idiopathic pericarditis, respectively. The molecule was approved in the US in March 2021 for the treatment of RP.
Huadong Medicine’s Licensing Deal and Market Potential
Huadong Medicine struck a USD 662 million licensing deal with Kiniksa in 2022, securing exclusive development, regulatory filing, and commercialization rights to rilonacept along with mavrilimumab, both autoimmune disease therapies, in China, South Korea, and other regions. Rilonacept was included in China’s first batch of clinically urgently needed drugs already approved overseas in 2018 and was awarded priority review status in 2023, highlighting its potential impact on patient care in the region.-Fineline Info & Tech