The National Medical Products Administration (NMPA) in China has conditionally approved amimestrocel from Beijing-based Platinum Life with priority review status. This mesenchymal stem cells (MSCs) therapy marks the first of its kind in China, now available for treating patients aged 14 and above with acute graft-versus-host disease (aGVHD) that primarily affects the digestive tract following the failure of hormone therapy.
Understanding Graft-Versus-Host Disease (GVHD)
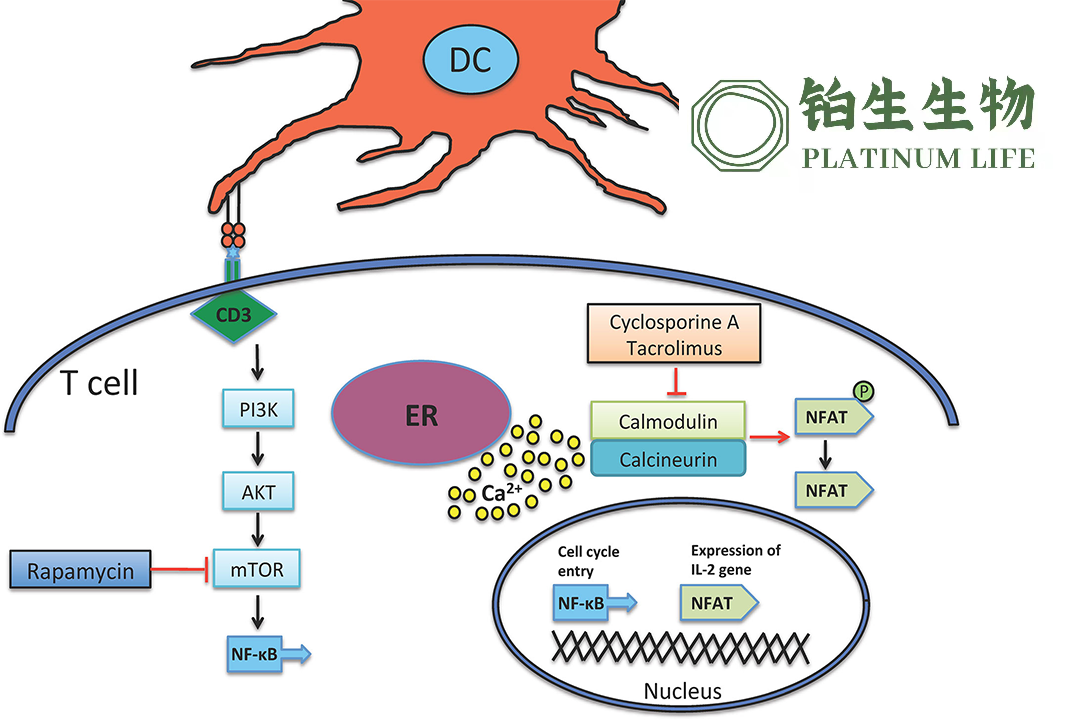
Graft-versus-host disease is a multi-organ syndrome that occurs post-allogeneic hematopoietic stem cell transplantation. In this condition, lymphocytes from the donor mount an attack on the recipient’s tissue. GVHD is characterized by tissue inflammation and fibrosis, affecting primarily the skin, gastrointestinal tract, liver, lungs, and mucosal surfaces.
The Impact of Amimestrocel’s Approval
The conditional approval of amimestrocel by the NMPA signifies a significant advancement in the treatment options for patients suffering from aGVHD in China. This therapy offers a new approach to managing the symptoms and complications associated with this challenging post-transplant condition, potentially improving outcomes for affected individuals.-Fineline Info & Tech