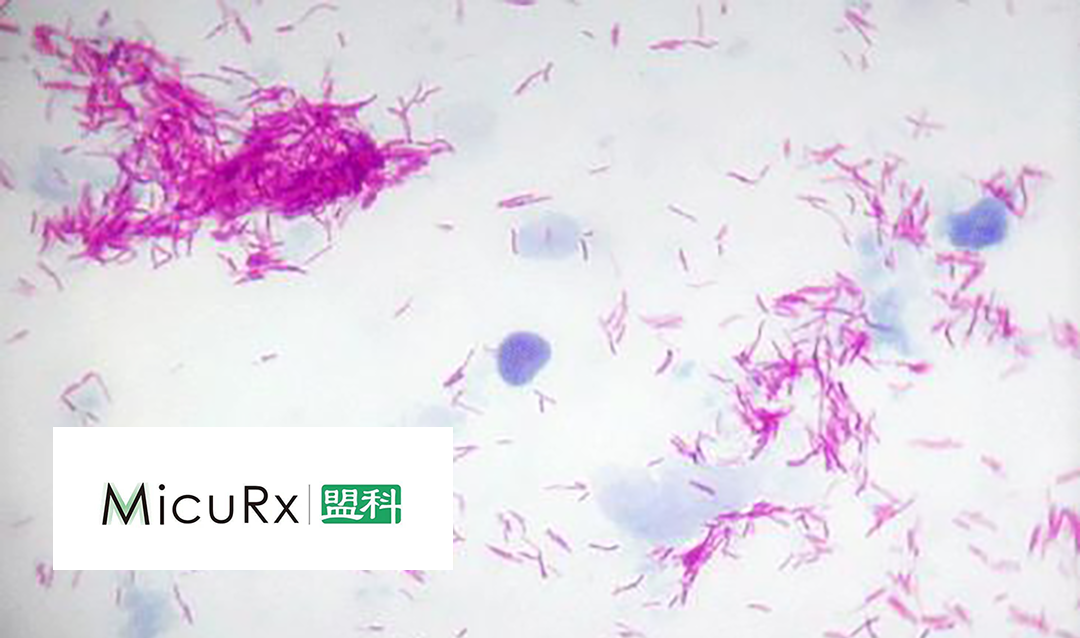
Sino-US firm MicuRx Pharmaceuticals Inc. (SHA: 688373) has announced that it has received approval from the National Medical Products Administration (NMPA) to conduct a clinical study for its drug MRX-5. The study will focus on infections caused by non-tuberculous Mycobacteria (NTM) that are sensitive to this product.
MRX-5: A Promising Benzodiazole Antibiotic for NTM Infections
MRX-5 is a novel benzodiazole antibiotic specifically designed to treat infections caused by Mycobacterium, particularly NTM, which is increasingly prevalent and has limited treatment options. The drug has demonstrated strong antibacterial activity in common NTM cases, offering a potential solution to this growing health concern.
Safety and Efficacy of MRX-5
In addition to its antibacterial properties, MRX-5 has shown good safety and pharmacokinetics in both animal and human studies. It also has minimal drug interactions and virtually no resistance, making it a promising candidate for oral administration in treating NTM infections. This approval by the NMPA marks a significant step forward in the development of new treatment options for patients affected by NTM.-Fineline Info & Tech