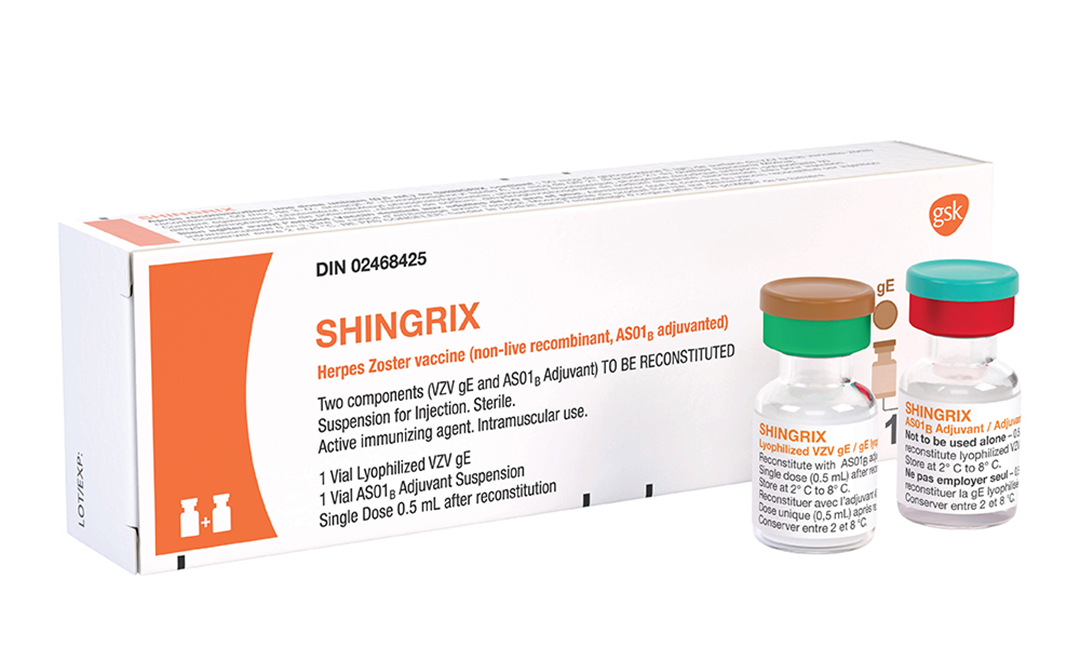
UK-headquartered GlaxoSmithKline (GSK; NYSE: GSK) has announced that a market filing for a prefilled syringe presentation of its recombinant zoster vaccine Shingrix has been accepted for review by the US Food and Drug Administration (FDA). This development marks a significant step towards enhancing the convenience and efficiency of vaccine administration.
New Presentation Details
The new prefilled syringe presentation of Shingrix removes the need to reconstitute separate vials prior to administration. This innovation offers a more convenient option for pharmacists, physicians, and other healthcare professionals who administer vaccinations. The new presentation maintains the same composition as the reconstituted vaccine, ensuring that the efficacy and safety profile remains consistent.
Benefits and Future Prospects
By eliminating the reconstitution step, the prefilled syringe presentation not only saves time but also reduces the potential for errors during the preparation process. This convenience factor is expected to improve the overall vaccination experience for both healthcare providers and patients. The acceptance of the market filing by the FDA is a crucial milestone, bringing GSK one step closer to making this improved presentation of Shingrix available to a broader audience.-Fineline Info & Tech