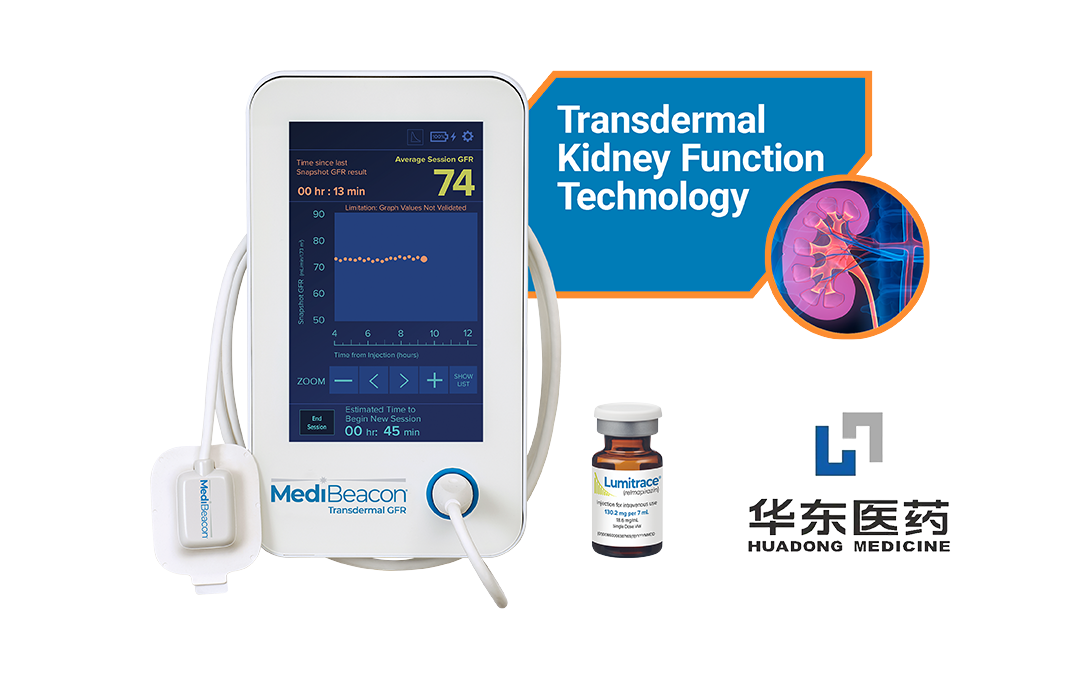
China-based Huadong Medicine Co., Ltd (SHE: 000963) has announced receiving marketing clearance from the US Food and Drug Administration (FDA) for its MediBeacon Transdermal GFR System (TGFR), a medical device co-developed with US partner MediBeacon Inc. The system is designed to evaluate renal function in adult patients with both impaired and normal kidney function.
MediBeacon TGFR: System Overview
The MediBeacon TGFR system comprises a monitor, sensors, and Lumitrace (relmapirazin), a non-radioactive, non-iodinated fluorescent GFR tracer. It assesses glomerular filtration rate (GFR) through non-invasive monitoring of fluorescence values of exogenous tracers over time. The system measures the in vivo clearance rate of fluorescent tracers and calculates real-time changes in Lumitrace fluorescence intensity via transdermal sensors placed on the skin surface. This method is unaffected by age, weight, gender, or race, making it a versatile tool for renal function assessment.
Commercialization and Market Reach
As the world’s first bedside product for renal function assessment in patients with normal or impaired kidney function, the MediBeacon TGFR system is being commercialized by Huadong Medicine in mainland China, Hong Kong, Taiwan, Singapore, Malaysia, and other regions in Asia. The FDA clearance marks a significant milestone in expanding the system’s global reach and enhancing its adoption in clinical settings.-Fineline Info & Tech