China-based Haisco Pharmaceutical Group Co., Ltd (SHE: 002653) has announced receiving clinical trial approval from the National Medical Products Administration (NMPA) for its investigational product HSK44459 in Behcet’s disease. This rare disease is listed in China’s second catalogue of rare diseases, highlighting the significant unmet medical need it represents.
Behcet’s Disease Background
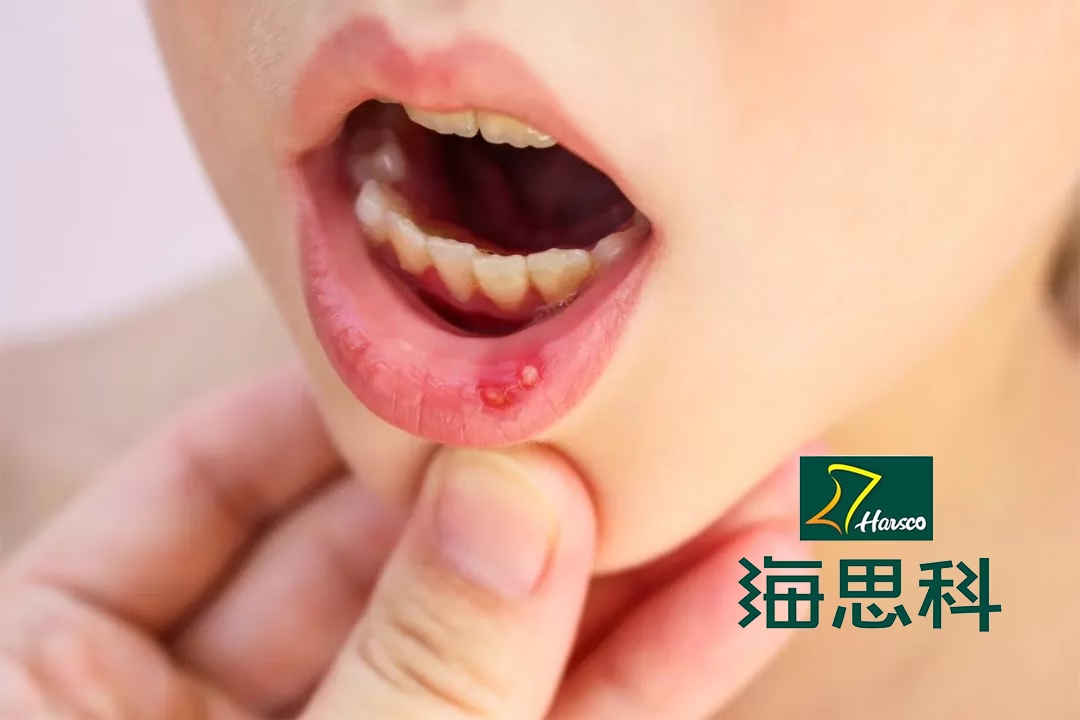
Behcet’s disease is a chronic, recurrent autoimmune/inflammatory condition characterized by vasculitis. It is primarily marked by recurrent oral and genital ulcers, uveitis, and skin damage, and can affect various organs including blood vessels, the heart, nervous system, gastrointestinal tract, joints, lungs, kidneys, and more. While no cure exists for the disease, apremilast—a PDE4 inhibitor—is approved for treatment, though it is associated with notable adverse reactions.
HSK44459: Development and Potential
Preclinical results indicate that HSK44459, a small molecule drug with a clear target and precise efficacy, offers good safety and a high benefit/risk ratio. This investigational product demonstrates significant potential for development and broad clinical application prospects. HSK44459 was previously cleared for study in interstitial lung disease in China in August of last year, further underscoring its therapeutic potential.-Fineline Info & Tech