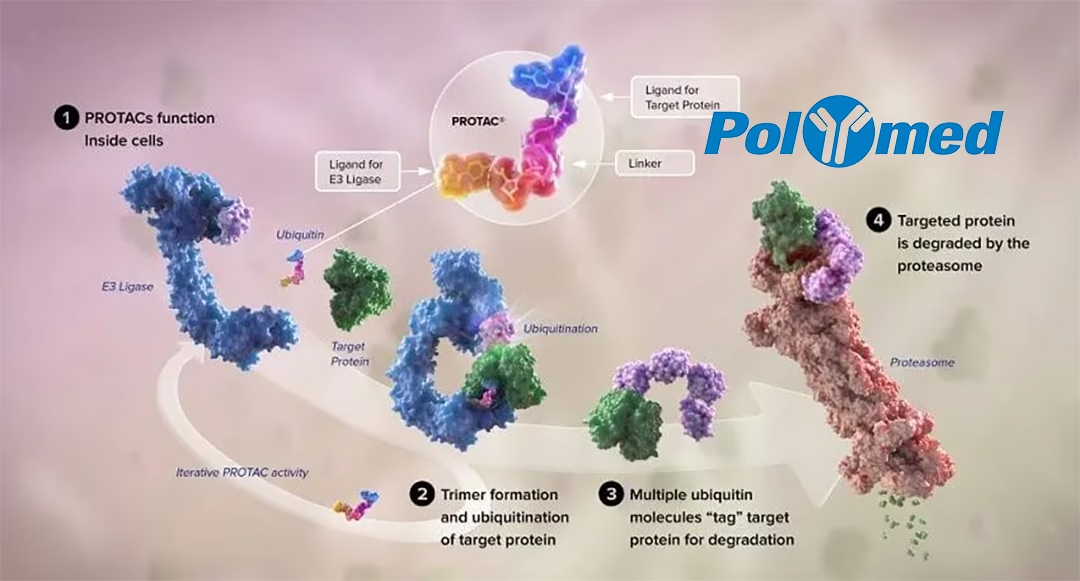
PolyMed Biopharma, a Hangzhou-based developer of Proteolysis Targeting Chimera (PROTAC) drugs, has entered into a licensing agreement with U.S.-based Photys Therapeutics, Inc. for its HPB-143, a targeted protein degrader (TPD) targeting IRAK4. Under the agreement, Photys will obtain exclusive global development, manufacturing, and commercialization rights to the drug, excluding Greater China and Southeast Asia.
Deal Terms
Photys will pay PolyMed an undisclosed upfront payment, milestone payments, and sales commissions. PolyMed will also acquire a partial equity stake in Photys.
Drug Profile
HPB-143 has higher oral bioavailability and weaker inhibition of the hERG ion channel in the heart compared to KT-474, another IRAK4-targeted TPD in Phase II trials. This indicates a higher potential for human cardiac safety and lower effective doses. HPB-143 has shown better efficacy in various mouse disease models.
Therapeutic Target
IRAK4 is being developed for the treatment of autoimmune diseases, including atopic dermatitis (AD) and hidradenitis suppurativa (HS). The drug is slated to enter Phase I trials this year.Fineline Info & Tech