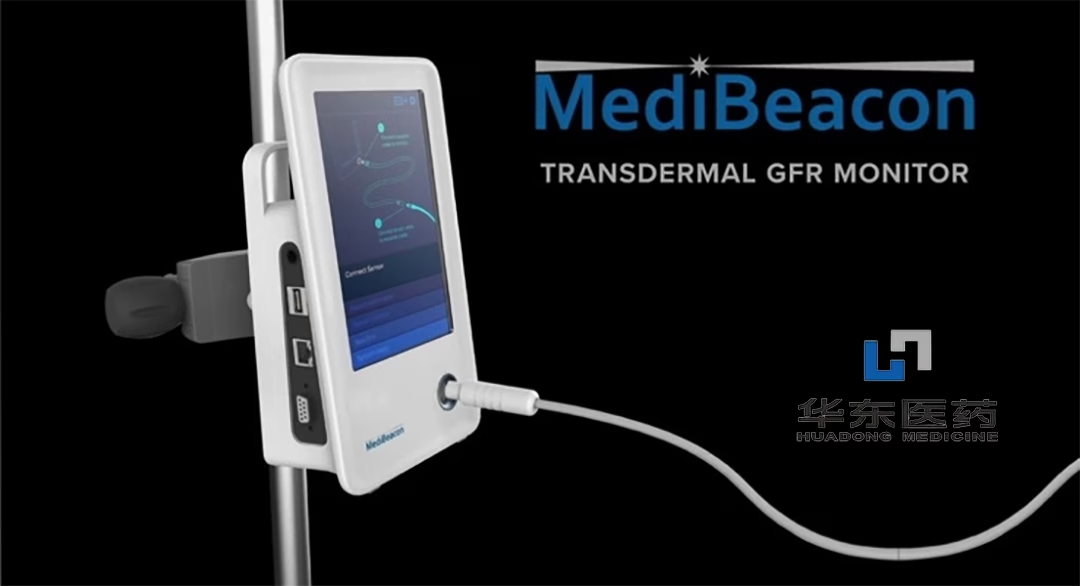
China-based Huadong Medicine Co., Ltd (SHE: 000963) announced that it has received marketing approval from the National Medical Products Administration (NMPA) for the MediBeacon Transdermal GFR System (TGFR), a Category III medical device licensed from US firm MediBeacon Inc. The device is designed to assess the glomerular filtration rate (GFR) of patients with 15ml/min/1.73m2 < GFR < 120ml/min/1.73m2, excluding those under dialysis, with anuria, or experiencing rapid changes in renal function. It is not intended for diagnosing acute kidney injury (AKI).
Product Profile
The TGFR system consists of a percutaneous glomerular filtration rate measurement device and lumitrace (relmapirazin), a non-radioactive, non-iodinated fluorescent GFR tracer. The device evaluates GFR in patients with impaired or normal renal function by noninvasively monitoring fluorescent light emission from an exogenous tracer agent over time.
Global Approval
MediBeacon’s TGFR system, the world’s first point-of-care product for renal function assessment in patients with normal or impaired kidney function, received approval in the US in January 2023. Huadong Medicine holds exclusive commercialization rights to the product in mainland China, Hong Kong, Taiwan, Singapore, Malaysia, and 25 other countries or regions in Asia.-Fineline Info & Tech