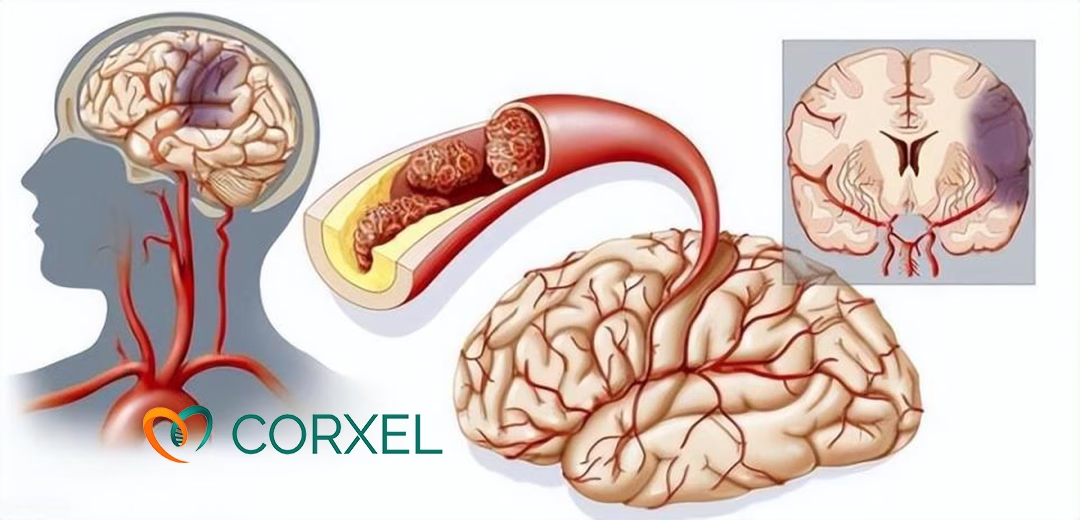
China-based Corxel Pharmaceuticals (CORXEL) has received approval from the National Medical Products Administration (NMPA) to conduct a clinical study evaluating the functional recovery of acute ischemic stroke (AIS) patients treated with JX10 within a time window of 4.5 to 24 hours from the “last known well.”
Drug Profile
JX10 is an investigational AIS drug candidate with both thrombolytic and anti-inflammatory properties. The drug aims to restore critical blood flow after acute stroke and extend the treatment window beyond the original 4.5-hour limit, potentially allowing more patients to benefit from standard treatments.
Clinical Study Details
The study will assess JX10’s efficacy in improving functional recovery for AIS patients treated within the specified time window, offering a potential breakthrough in stroke treatment.-Fineline Info & Tech