China-based biotech Akeso Inc. (HKG: 9926) announced the completion of patient enrollment in the Phase I clinical study assessing the safety, tolerability, pharmacokinetics, and preliminary efficacy of its AK138D1 in advanced malignant tumors in Australia.
Drug Profile
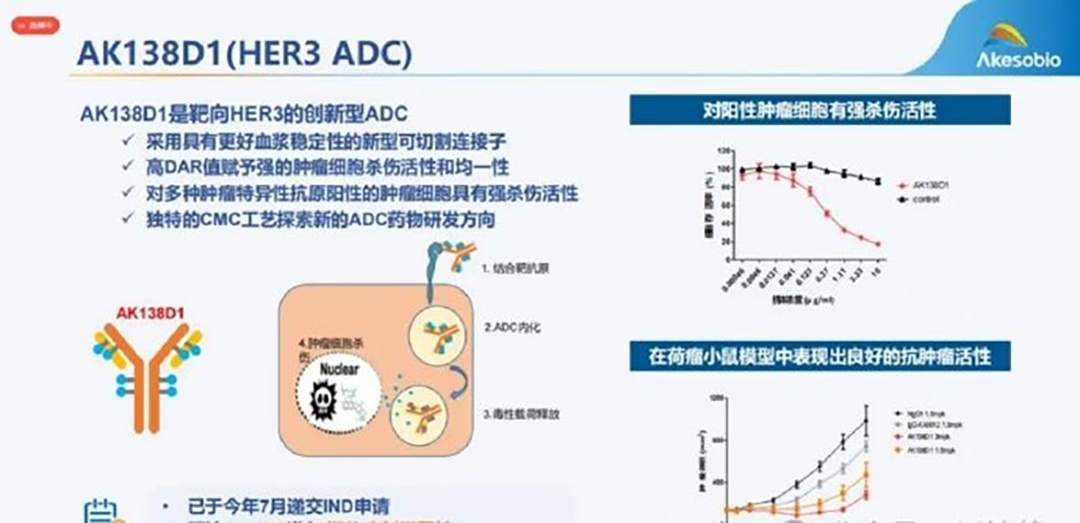
AK138D1, an antibody-drug conjugate (ADC) targeting human epidermal growth factor receptor 3 (HER3), features a fully humanized anti-HER3 IgG1 antibody, patritumab. It is conjugated to the topoisomerase I inhibitor DXd through a cleavable linker, MC-AAA (maleimide-alanine-alanine-alanine). After binding to HER3 on tumor cells, the ADC is internalized into the tumor cells, where the linker is cleaved, releasing the membrane-permeable DXd, a process leading to DNA damage and subsequent cell apoptosis.-Fineline Info & Tech