China-based Sino Medical Sciences Technology Inc., (SHA: 688108) announced that it has received marketing approval from the Ministry of Health of Vietnam for its HT Supreme drug-eluting stent system. The approval allows the product to be used to improve the diameter of the coronary artery lumen in symptomatic heart disease patients caused by primary coronary artery lesions (length ≤ 40 millimeters), with a reference vessel diameter of 2.25-5.00 millimeters.
Product Profile
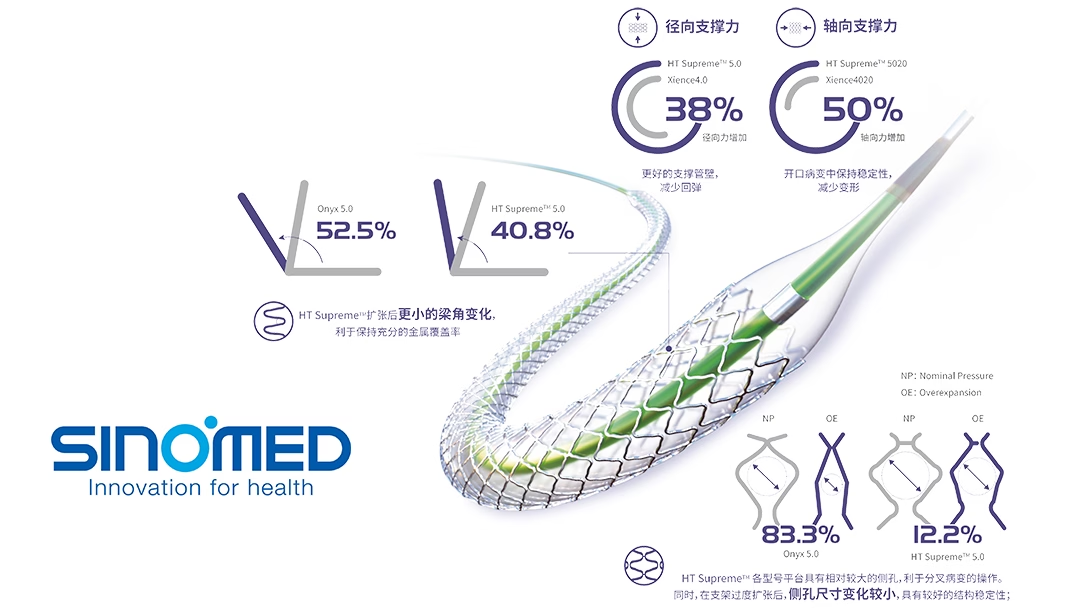
The HT Supreme drug-coated coronary artery stent system is a drug-device combination product. It consists of a drug-coated expandable cobalt-chromium (CoCr) alloy coronary artery stent and a balloon delivery system.-Fineline Info & Tech