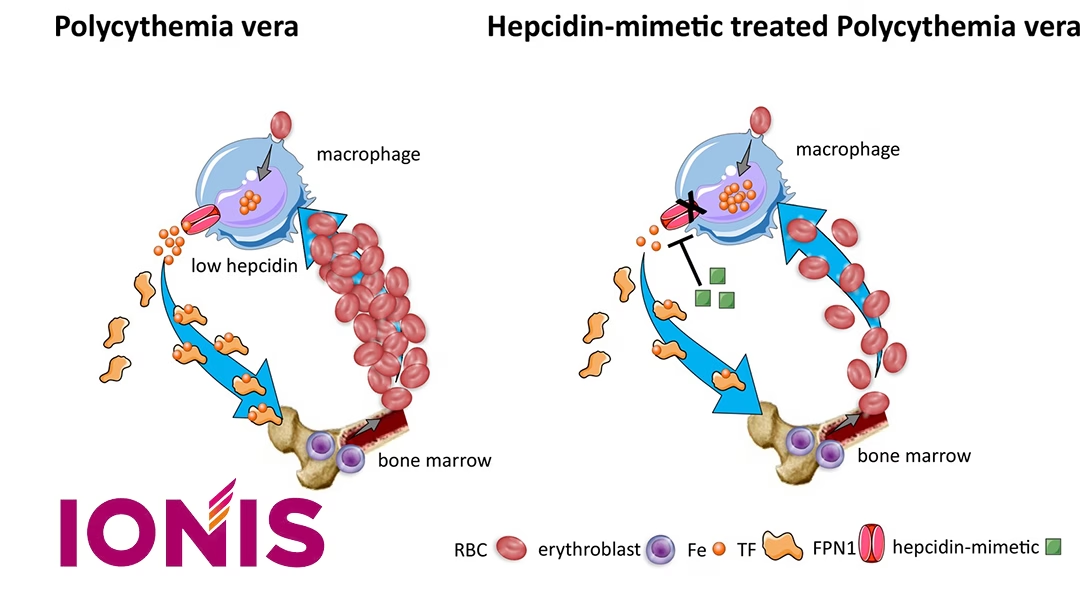
Japan-based Ono Pharmaceutical Co., Ltd. (TYO: 4528) announced an agreement with Ionis Pharmaceuticals, Inc. to license sapablursen, an investigational RNA-targeted medicine for the treatment of polycythemia vera (PV). Under the terms of the agreement, Ono has secured global development and commercialization rights to the drug.
Terms of the Agreement
Ono will pay Ionis an upfront fee of USD280 million and has committed to up to USD660 million in additional payments based on the achievement of development, regulatory, and sales milestones. Additionally, Ono will pay royalties in the mid-teens on annual net sales of sapablursen.
Development and Regulatory Responsibilities
Ionis will remain responsible for completing the ongoing Phase II IMPRSSION study, while Ono will take full charge of subsequent development, regulatory filings, and commercialization efforts. Sapablursen has been granted Fast Track designation and orphan drug designation by the U.S. Food and Drug Administration (FDA) in January 2024 and August 2024, respectively.
Clinical Trial Status
Sapablursen is currently being assessed in the fully enrolled Phase II IMPRSSION study in adult patients with PV. The drug shows promise in addressing a significant unmet medical need in this patient population.-Fineline Info & Tech