Simcere Pharmaceutical Group Limited (HKG: 2096) and AnDiConBio announced that the New Drug Application (NDA) for ADC189, their co-developed anti-influenza drug, has been accepted for review by China’s National Medical Products Administration (NMPA). The drug is intended for treating uncomplicated influenza A and B in adults and adolescents.
Mechanism and Efficacy
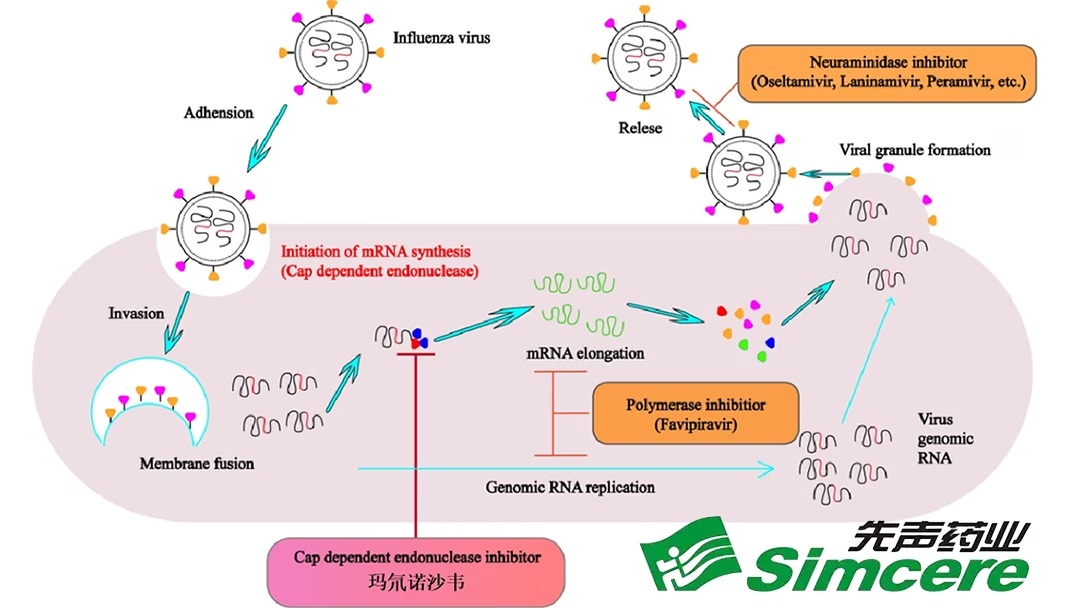
ADC189 is a cap-dependent endonuclease inhibitor that blocks the transcription of viral mRNA, rendering the influenza virus unable to replicate. Phase II/III study results showed a 26.543% improvement in median relief time for all influenza symptoms compared to the placebo group, with a statistically significant difference (P<0.0001). The safety profile was consistent with the control group.
Unique Treatment Advantage
Notably, ADC189 requires only a single oral dose throughout the entire treatment course and can prevent influenza virus replication within 24 hours, achieving a “one-day negative conversion.”
Previous Clinical Progress
The Phase III study for ADC189 in children aged 2-11 with influenza has completed clinical enrollment and follow-up. Additionally, the drug received tacit clinical approval for post-exposure prophylaxis of influenza A and B in individuals aged 2 and above.-Fineline Info & Tech