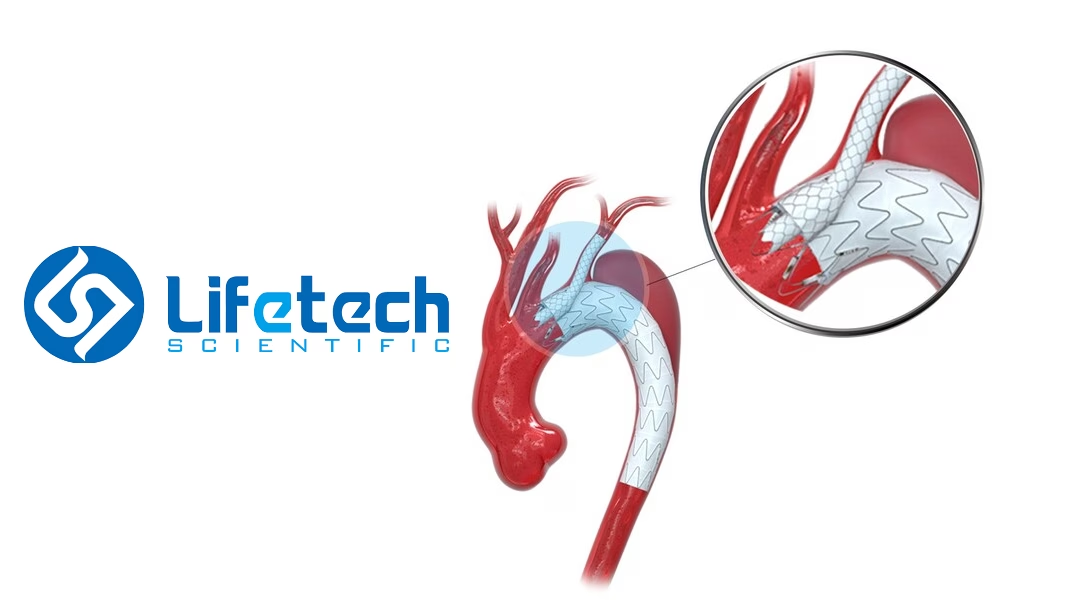
China-based Lifetech Scientific Corporation (HKG: 1302) announced receiving market approval from the National Medical Products Administration (NMPA) for its aortic branch reconstruction covered stent system (chimney), co-developed with Fuwai Hospital. This innovative product offers a novel treatment option for patients with arch lesions.
Product Innovation
The aortic branch covered stent system is the world’s first designed to reconstruct arch branches via chimney technique. Comprising the Longuette skirt edge stent (long) and Ankura Pro aortic body covered stent system, it provides a minimally invasive solution for complex aortic arch lesions.
Clinical Validation
The product demonstrated safety and efficacy in a prospective, multicenter, single-group target value method pre-market clinical study involving 150 patients in China. It showed promising results in treating Stanford B-type dissection lesions near the left subclavian artery.-Fineline Info & Tech