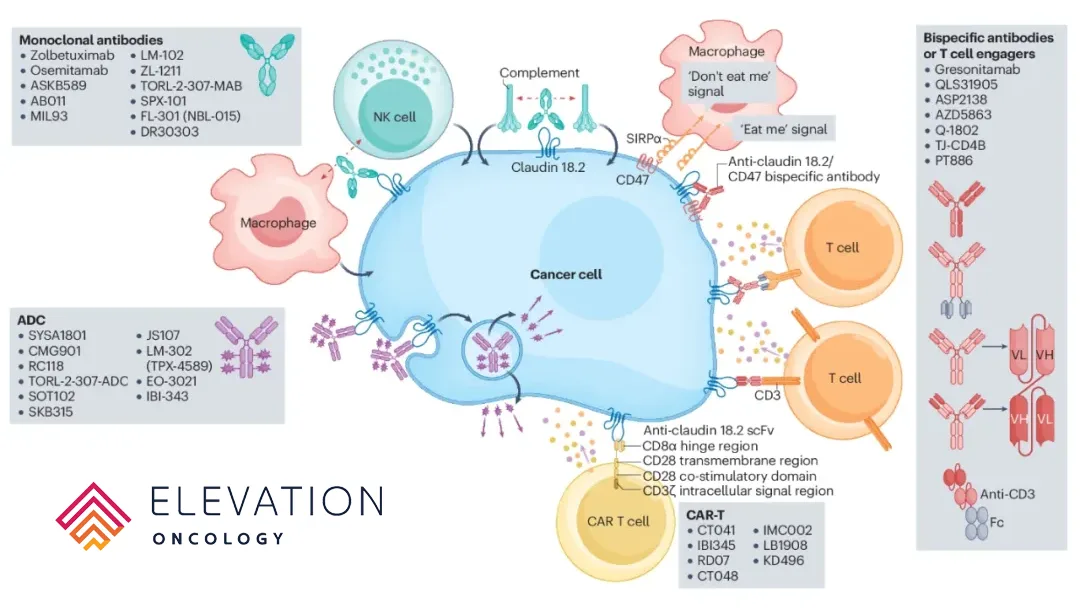
US-based Elevation Oncology, Inc. (NASDAQ: ELEV) has announced the decision to terminate development of EO-3021 following disappointing Phase 1 data, which caused its stock price to plummet 41% before trading.
EO-3021’s Background and Licensing Deal
EO-3021, a Claudin 18.2-targeted antibody drug conjugate (ADC) originated by China’s CSPC Pharmaceutical Group Ltd (HKG: 1093), was licensed by Elevation for ex-Greater China rights in a USD 1.195 billion licensing deal signed between the two companies in July 2022.
Phase 1 Trial Results and Decision to Halt Development
The drug delivered uncompetitive efficacy data in Phase I trials compared with other similar drugs, although its safety profile was good. This led to the decision to halt further development of EO-3021.
Shift to EO-1022 Development
Elevation will instead move on to develop EO-1022, an HER3 ADC utilizing Synaffix’s glycan site-specific conjugation and linker-payload technology, marking a strategic shift in the company’s pipeline focus.-Fineline Info & Tech