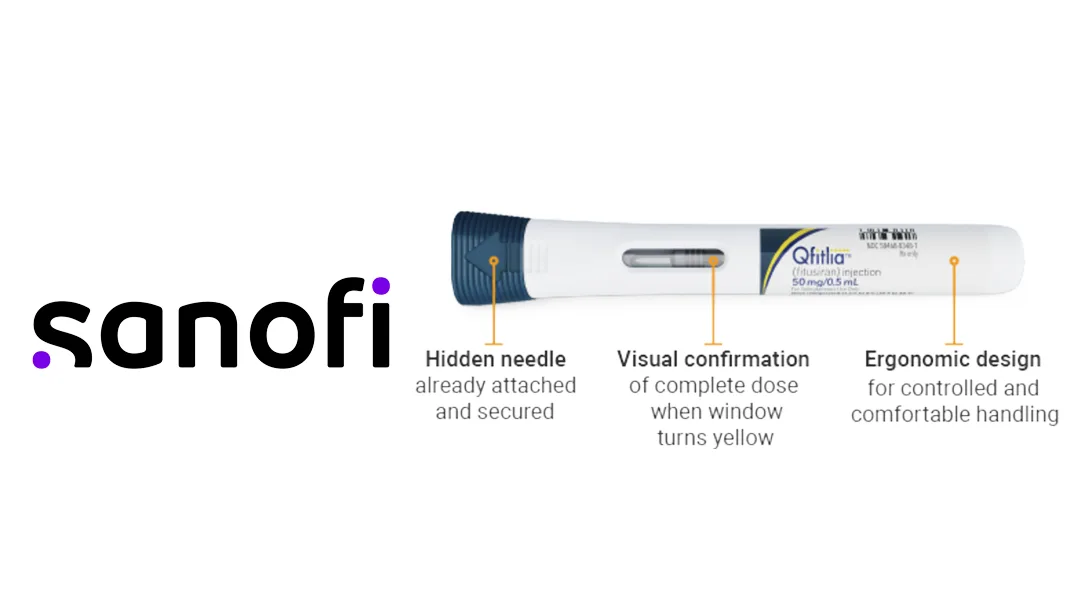
France-based Sanofi (EPA: SAN, NASDAQ: SNY) has announced receiving marketing approval from the US Food and Drug Administration (FDA) for Qfitlia (fitusiran). This antithrombin-lowering (AT) therapy is now approved for routine prophylaxis to prevent or reduce bleeding episodes in adult and pediatric patients (aged 12 or older) with hemophilia A or B, with or without factor VIII or IX inhibitors.
Innovative Therapy Mechanism
Qfitlia is a first-in-class AT-lowering therapy for the prophylactic treatment of hemophilia A and B. It leverages small interfering RNA (siRNA) technology to achieve low-frequency administration, requiring as few as six subcutaneous injections per year. This innovation addresses the need for less frequent and more convenient treatment options for hemophilia patients.
Clinical Trial Success
The FDA approval is supported by positive results from the Phase III ATLAS study, which demonstrated clinically meaningful protection against bleeds. The study showed significant reductions in annualized bleeding rates (ABR) across hemophilia patients, both with and without inhibitors.
Global Development and Companion Diagnostic
In China, Qfitlia was included in the patient-centered rare disease drug development pilot program (Care Plan) in November 2024, with regulatory decisions expected later this year. Additionally, Innovance, an antithrombin assay by Siemens Healthineers AG, received FDA clearance as a companion diagnostic for Qfitlia, enabling precise measurement of AT levels to guide therapy.-Fineline Info & Tech