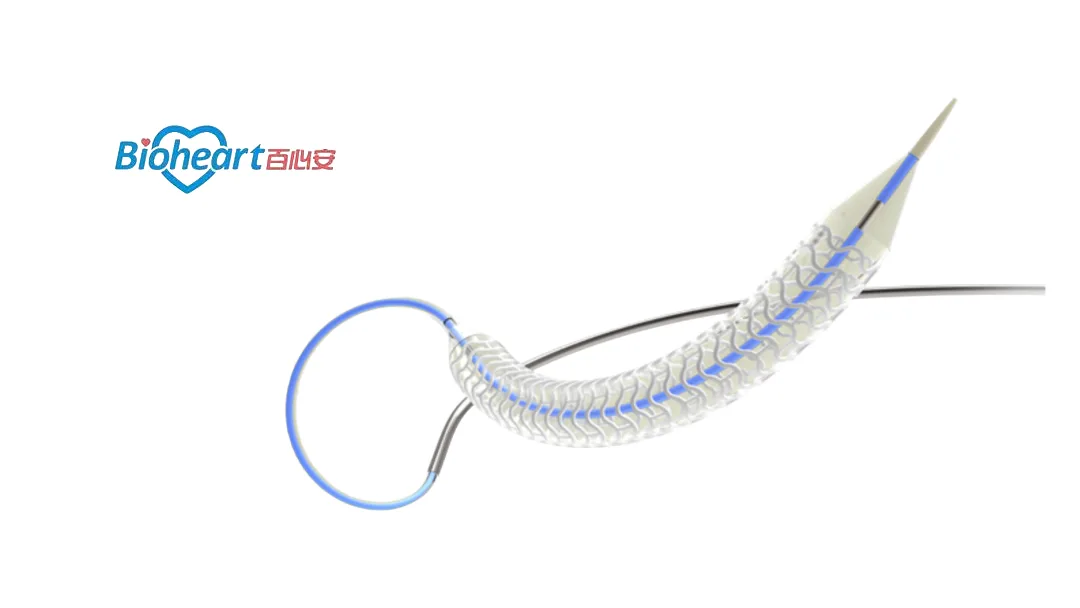
China-based Shanghai Bio-heart Biological Technology Co., Ltd (HKG: 2185) announced the first patient enrollment for the SAKURA-SCB study at the Cardiovascular Institute in Tokyo, Japan. This single-blinded, multiple comparison study aims to evaluate the efficacy and safety of the company’s investigational drug-coated balloon (DCB) with rapamycin.
DCB Innovation
The DCB, designed for treating neovascular small vessel and stent restenosis, utilizes a rapamycin drug-eluting balloon catheter. It incorporates rapamycin, amphiphilic liposomes, biodegradable polymer sustained-release agents, and dispersants, ensuring efficient drug transfer and sustained release.
Advantages Over Existing Products
Unlike current DCB products in Japan, which use paclitaxel-based coatings, Bio-heart’s DCB leverages rapamycin’s anti-inflammatory properties and unique cell inhibitory effects. This offers enhanced safety, a broader therapeutic window, and reduced restenosis risk, positioning it as a promising alternative in vascular intervention.-Fineline Info & Tech