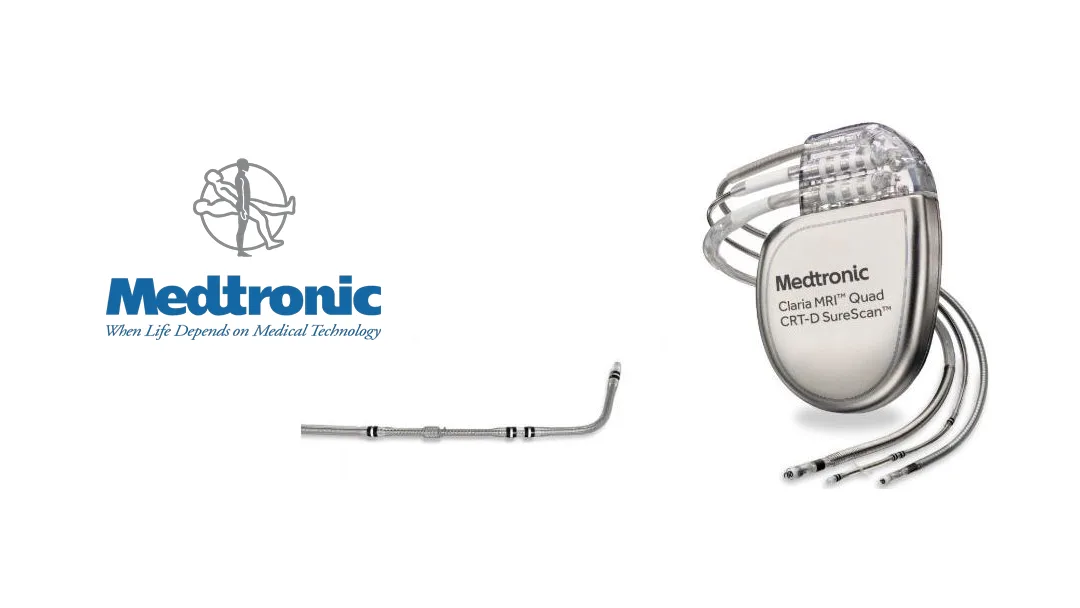
China’s National Medical Products Administration (NMPA) has approved Medtronic’s (NYSE: MDT) endovascular implantable defibrillation electrode lead and extravascular implantable defibrillation electrode lead introducer. These components are designed to be used with an external implantable defibrillator to form a comprehensive system for patients at risk of life-threatening ventricular tachycardia.
Product Features and Benefits
The approved products are placed under the sternum and offer automatic treatment for patients at significant risk of developing ventricular tachycardia. The system includes cardiac and extravascular anti-tachycardia pacing functions and arrest prevention pacing, reducing complications associated with intravenous leads. Additionally, it meets the clinical needs of patients requiring MRI examinations with 1.5T and 3.0T field strengths.
Market and Clinical Impact
This approval highlights Medtronic’s commitment to advancing medical technology in China and addresses the growing demand for safe and effective implantable defibrillator systems. The new system offers improved patient outcomes and enhanced compatibility with imaging technologies.-Fineline Info & Tech