Kyowa Kirin (TYO: 4151) announced it will cancel its licensing agreement with Amgen (NASDAQ: AMGN) for rocatinlimab, an anti‑OX40 monoclonal antibody, regaining global rights to the asset. The drug, discovered in collaboration with the La Jolla Institute for Immunology, represents a core strategic priority for Kyowa Kirin’s future pipeline.
Deal Structure & Strategic Rationale
| Item | Detail |
|---|---|
| Licensor | Amgen (Nasdaq: AMGN) |
| Licensee (Returning) | Kyowa Kirin (TSE: 4151) |
| Drug | Rocatinlimab (anti‑OX40 monoclonal antibody) |
| Action | Licensing agreement cancellation |
| Rights Reversion | Global rights return to Kyowa Kirin |
| Discovery Partner | La Jolla Institute for Immunology |
| Strategic Priority | Core asset for Kyowa Kirin’s future pipeline |
Drug Profile & Innovation
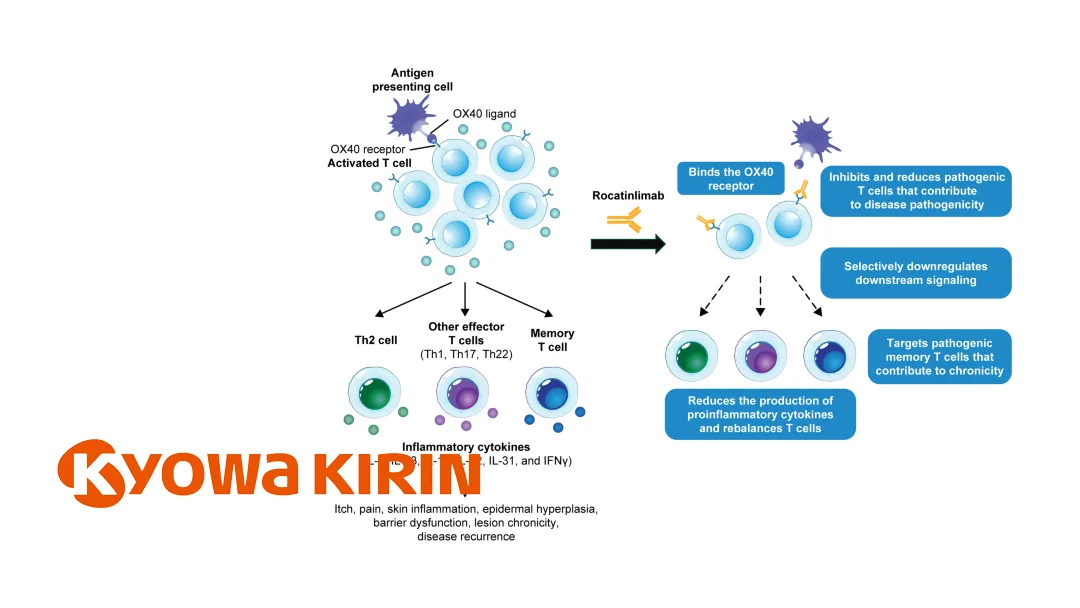
- Mechanism: Anti‑OX40 monoclonal antibody designed for T‑cell rebalancing therapy
- Unique Approach: Inhibits and reduces pathogenic effector and memory T cells by targeting the OX40 receptor
- Market Position: Potential first‑in‑class “T‑cell rebalancing” therapy
- Strategic Value: Represents cornerstone of Kyowa Kirin’s clinical and commercial strategy in immunology
Market Impact & Commercial Outlook
- Autoimmune Market: Global autoimmune market valued at $150 billion : in 2025; T‑cell targeted therapies gaining traction
- Competitive Landscape: Rocatinlimab would compete with JAK inhibitors and other biologics; OX40 mechanism offers differentiation
- Strategic Shift: Reclaiming global rights allows Kyowa Kirin to control development timeline, pricing, and commercial strategy in key markets
- Development Plans: Kyowa Kirin will leverage its clinical and commercial expertise to advance rocatinlimab independently
- Financial Impact: Transaction eliminates future milestone/royalty obligations to Amgen, improving long‑term profitability
- Next Steps: Updated clinical development plan expected Q2 2026; potential partnership discussions for ex‑Japan rights under evaluation
Forward‑Looking Statements
This brief contains forward‑looking statements regarding development timelines, commercial strategy, and market potential for rocatinlimab. Actual results may differ due to clinical trial outcomes, competitive dynamics, and regulatory review processes.