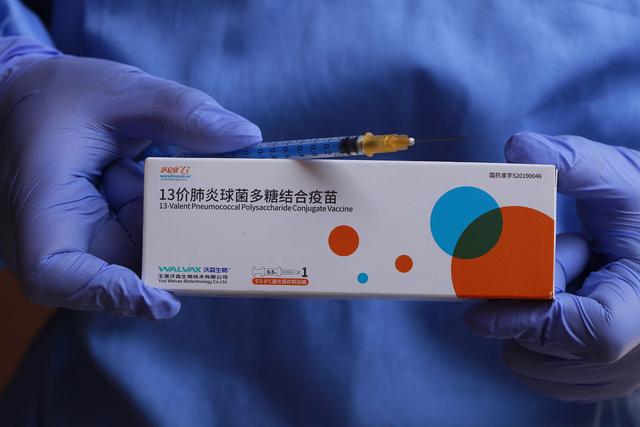
Walvax Biotechnology Co., Ltd (SHE: 300142), a Chinese biopharmaceutical company, has announced that it has received market approval in the Sultanate of Oman for its vaccine Weuphoria, a 13-valent pneumococcal polysaccharide conjugate vaccine (PCV-13). This approval marks a significant step for the company as it expands the reach of its vaccine portfolio internationally.
Weuphoria’s PCV-13 vaccine is designed to effectively protect infants and young children aged from 6 weeks to 5 years against infections caused by 13 serotypes of Streptococcus pneumoniae, including Types 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. By improving immunity, the vaccine offers efficient protection to children in Oman, contributing to the country’s public health efforts.
The approval in Oman highlights Walvax Bio’s commitment to developing and providing vaccines that address global health needs and underscores the potential for the company’s products to make a significant impact in international markets.- Flcube.com