Dec. 2024 Fineline Deals Book
China Pharmaceutical BD Transaction Review – Deeply empowering pharmaceutical transactions to facilitate collaboration and jointly build a new ecosystem for BD communication. – Fineline Deals Book Dec. 2024
According to incomplete statistics from Fineline Info & Tech, there were a total of 25 pharmaceutical BD and M&A transactions in China in December 2024, including 4 cross-border asset acquisitions, 13 outbound transactions, and 8 domestic transactions.

Cross-border License In
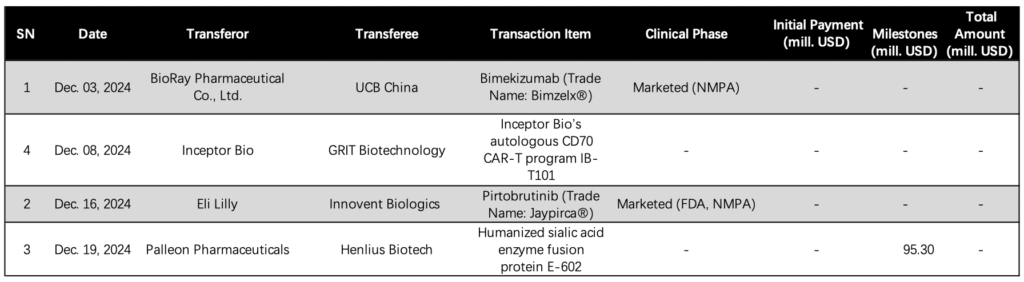
UCB Partners with BioRay Biopharmaceutical for Bimzelx Commercial Promotion in China
Belgium based UCB (EBR: UCB) is fulfilling its commitment to enhance attention to innovative products and local partnerships in the Chinese market by signing an agreement with Zhejiang based BioRay Pharmaceutical Co., Ltd. for the commercial promotion of its Bimzelx (bimekizumab) in China. The financial details of the deal have not been disclosed. This partnership follows UCB’s recent divestment of its mature neurology and allergy business in China to CBC Group and Mubadala Investment Compa. [Full Artical]
Innovent Biologics Partners with Eli Lilly for Distribution of Jaypirca in China
China-based Innovent Biologics, Inc. (HKG: 1801) has entered into a significant distribution and promotion agreement with US pharmaceutical giant Eli Lilly and Company (NYSE: LLY). The agreement pertains to Lilly’s non-covalent Bruton’s tyrosine kinase (BTK) inhibitor, Jaypirca (pirtobrutinib). Financial details of the agreement have not been disclosed. Under the terms, Innovent will handle the importation, marketing, distribution, and promotion of Jaypirca in mainland China, while Lilly will maintain responsibility for the drug’s R&D and post-market medical affairs. [Full Artical]
Henlius Biotech Expands Partnership with Palleon Pharmaceuticals for Autoimmune Disease Treatment
Shanghai Henlius Biotech Inc. (HKG: 2696), a leading biotechnology company based in China, has announced an expansion of its alliance with Palleon Pharmaceuticals Inc. This partnership, originally established in June 2022, now includes the US firm in the development and commercialization of a combination therapy using Palleon’s E-602 and Henlius’ HanLiKang (rituximab) for patients with autoimmune diseases, including lupus nephritis (LN). [Full Artical]
Inceptor Bio and GRIT Biotechnology Partner to Advance CAR-T Therapy IB-T101 for Solid Tumors
US-based Inceptor Bio has entered into a partnership with Chinese firm GRIT Biotechnology to advance the potential best-in-class chimeric antigen receptor (CAR)-T therapy, IB-T101, for the treatment of solid tumors. This collaboration combines the expertise of two cell therapy specialists to enhance the development and commercialization of innovative cancer treatments. [Full Artical]
Cross-border License Out
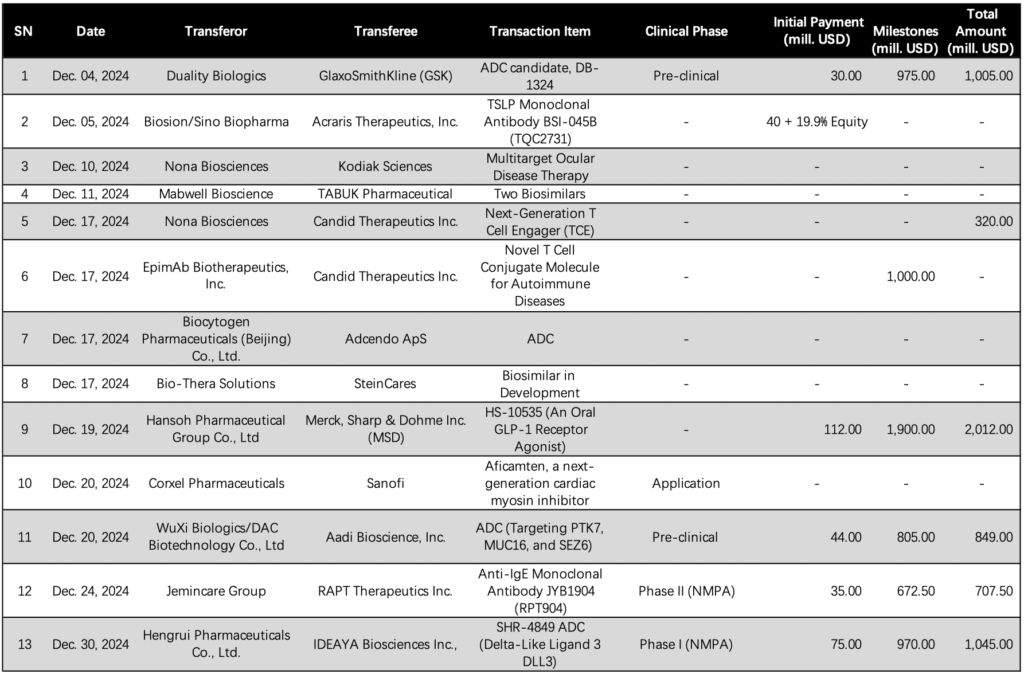
GSK plc Enters Exclusive Option Agreement with Duality Biologics for ADC Candidate DB-1324
UK-based GSK plc (NYSE: GSK) has entered into an exclusive option agreement with Duality Biologics, a developer of antibody conjugate drugs (ADCs) with operations in the United States and China. The agreement is in relation to a potentially best-in-class ADC candidate, DB-1324. [Full Artical]
Biosion Secures Licensing Deal with Aclaris Therapeutics for Global Rights to BSI-045B and BSI-502
Biosion, a clinical stage biotech company with operations in Delaware, US, and China, has entered into a licensing agreement with US based Acraris Therapeutics, Inc. (NASDAQ: ACRS). The deal grants Acraris exclusive global rights, excluding Greater China, to Biosion’s BSI-045B and BSI-502, further strengthening the two companies’ layout in the field of immunomodulatory therapy.[Full Artical]
Mabwell Bioscience Secures Licensing Deal with TABUK Pharmaceutical for MENA Region
China-based Mabwell (Shanghai) Bioscience Co., Ltd. (SHA: 688062) has inked a licensing deal with TABUK Pharmaceutical Manufacturing Company, granting the Saudi Arabian company the regulatory filing and commercialization rights to two of Mabwell’s biosimilars in the Middle East and North Africa (MENA) region. The financial details of the agreement were not disclosed.[Full Artical]
Adcendo ApS Expands Partnership with Biocytogen to Enhance ADC Pipeline
China’s Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (HKG: 2315) has announced that Denmark-based Adcendo ApS has exercised its options within a research, option, and licensing agreement to utilize Biocytogen’s fully human antibodies. This move is aimed at bolstering Adcendo’s antibody drug conjugate (ADC) pipeline. [Full Artical]
Bio-Thera Solutions Expands License Deal with SteinCares for Latin American Market
China-based Bio-Thera Solutions (SHA: 688177) has announced the addition of a third biosimilar to its existing license agreement with Costa Rican partner SteinCares, which was initially struck in March of this year. Under the revised accord, SteinCares will gain exclusive rights to distribute and market the new biosimilar in Brazil and across the Latin American (LatAm) region. Financial details of the agreement have not been disclosed. [Full Artical]
Sanofi and Corxel Pharmaceuticals Partner for Aficamten in Greater China Rights
Global healthcare leader Sanofi (NASDAQ: SNY) and China-based Corxel Pharmaceuticals (CORXEL), formerly known as Ji Xing Pharmaceuticals, have jointly announced an official agreement. Under this partnership, Sanofi is set to acquire exclusive development and commercialization rights to CORXEL’s aficamten in Greater China. The financial details of the deal have not been disclosed, but it is expected to close within this year. [Full Artical]
WuXi Biologics Enters Licensing Deal with Hangzhou DAC and Aadi Bio for Preclinical ADC Portfolio
China-based Contract Research and Development Organization (CRDMO) WuXi Biologics (HKG: 2269) has announced a strategic licensing agreement with compatriot firm Hangzhou DAC Biotechnology Co., Ltd, an antibody drug conjugate (ADC) specialist, and US-based Aadi Bioscience, Inc. (NASDAQ: AADI). The deal grants Aadi Bio global d2018evelopment and commercialization rights to a three-asset portfolio of preclinical ADCs. [Full Artical]
RAPT Therapeutics Secures Ex-China Rights to Jemincare Group’s Anti-IgE Antibody JYB1904
US-based biopharmaceutical company RAPT Therapeutics Inc. (NASDAQ: RAPT) has announced that it has obtained the ex-Greater China rights to Jiangxi Jemincare Group’s long-acting anti-IgE antibody, JYB1904, through a significant licensing agreement. The deal involves an upfront payment of USD 35 million and potential milestone payments of up to USD 672.5 million, in addition to sales royalties.[Full Artical]
Hengrui Pharmaceuticals and IDEAYA Biosciences Ink Licensing Deal for SHR-4849
Jiangsu Hengrui Pharmaceuticals Co., Ltd. (SHA: 600276) has announced a significant licensing agreement with US-based IDEAYA Biosciences Inc., (NASDAQ: IDYA). Under this agreement, Hengrui will grant IDEAYA development, manufacturing, and commercialization rights to its novel DLL3-targeting Topo-I-payload antibody drug conjugate (ADC), SHR-4849, for markets worldwide outside of Greater China. [Full Artical]
Domestic Transactions in China
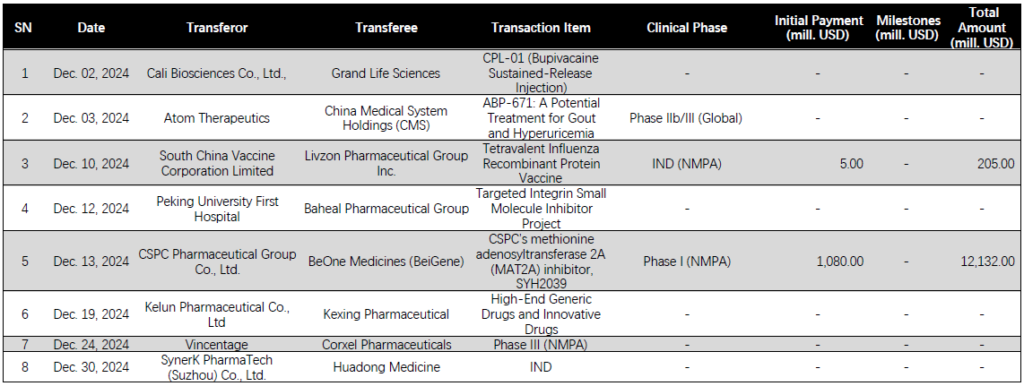
Grand Life Sciences Partners with Cali Biosciences for CPL-01 Commercialization in China
China-based Grand Life Sciences has entered into a partnership with compatriot firm Cali Biosciences Co., Ltd., becoming the exclusive commercialization partner for CPL-01 (ropivacaine sustained-release injection) in China. Under the agreement, Grand Life Sciences will pay Cali Biosciences an undisclosed amount of upfront and milestone payments, while Cali Biosciences will pay Grand Life Sciences market promotion service fees. [Full Artical]
China Medical System Holdings Secures Exclusive Rights to Atom Therapeutics’ Gout Drug ABP-671
China Medical System Holdings (CMS; HKG: 0867) has entered into a licensing agreement with compatriot firm Atom Therapeutics, securing exclusive commercialization rights to ABP-671, a Category 1 chemical drug for gout and hyperuricemia, in mainland China, Hong Kong, and Macau. The financial specifics of the agreement were not disclosed. [Full Artical]
PKU 1st Hospital Partners with Baheal Pharmaceutical on Integrin-Targeted Inhibitor Project
Peking University First Hospital (PKU 1st Hospital) has partnered with China-based health services and distribution giant Baheal Pharmaceutical Group for its integrin-targeted small molecule inhibitor project. The deal, valued at RMB 70 million (USD 9.5 million), grants Baheal exclusive global rights for patent, clinical development and regulatory filing, manufacturing, and market sales of the program.[Full Artical]
BeiGene Ltd Acquires Global Rights to CSPC’s MAT2A Inhibitor SYH2039
BeiGene Ltd (NASDAQ: BGNE, HKG: 6160, SHA: 688235) has entered into a significant licensing agreement with CSPC Pharmaceutical Group Co., Ltd. (HKG: 1093). The agreement secures BeiGene the global development, manufacturing, and commercialization rights to CSPC’s methionine adenosyltransferase 2A (MAT2A) inhibitor, SYH2039. [Full Artical]
Kexing and Kelun Partner to Expand Overseas Business for Generic Drugs and Therapies
China-based firms Kexing Pharmaceutical (SHA: 688136) and Sichuan Kelun Pharmaceutical Co., Ltd (SHE: 002422) have agreed to collaborate on the overseas business development of Kelun’s high-end generic drugs and innovative therapies. The partnership aims to leverage the strengths of both companies to expand their global footprint. [Full Artical]
China-based Corxel Pharmaceuticals (CORXEL) has announced a significant licensing agreement with domestic firm Vincentage, securing global development and commercialization rights to CX11 (also known as VCT220), an oral small molecule glucagon-like peptide-1 receptor agonist (GLP-1 RA), excluding Greater China. The financial details of the agreement have not been disclosed. [Full Artical]
Huadong Medicine Partners with SynerK PharmaTech for Hypertension siRNA Drug Development
China-based Huadong Medicine Co., Ltd (SHE: 000963) has announced a strategic partnership agreement with SynerK PharmaTech (Suzhou) Co., Ltd., an RNA-targeted therapies developer with operations in Boston, US, and Suzhou and Beijing, China. The collaboration aims to develop an angiotensinogen (AGT)-targeted small nucleic acid (siRNA) drug for the treatment of hypertension, with Huadong securing the exclusive option for development, registration, manufacturing, and commercialization rights in Greater China. [Full Artical]
Monthly Roundup
According to the partial statistics from Fineline Info & Tech, by December 2024, China’s biopharmaceutical sector has achieved remarkable success in the transaction domain, completing a total of 229 deals. These transactions include 35 cross-border introductions, 111 cross-border exports, and 83 domestic transactions within China. In December alone, the industry was particularly vibrant, with 25 deals concluded, consisting of 4 cross-border introductions, 13 cross-border exports, and 8 domestic transactions. The business development (BD) transactions in China’s pharmaceutical sector this month are characterized by the following notable features:
- There were 4 cross-border introduction transactions, 2 of which involved the introduction of products from multinational pharmaceutical companies in China (BioRay Pharmaceutical Bio and UCB, Innovent Biologics and Eli Lilly), and the other 2 were for the introduction of early-stage projects (Henlius Biotech and Palleon Pharmaceuticals, GRIT Biotechnology and Inceptor Bio). Chinese companies fully leveraged their commercialization and R&D capabilities, achieving synergistic empowerment.
- There were 13 authorized export transactions in December, with international pharmaceutical giants such as GSK, MSD, and Sanofi continuing to purchase high-quality Chinese assets. In terms of product types, Hansoh Pharma’s GLP-1 deal with MSD reached $2 billion, and ADC molecules from Hengrui Pharmaceuticals, DAC Biotechnology, and Biocytogen remained active. Candid focused on the new favorite T-cell engagers of the year, completing two transactions. It is worth mentioning that Corxel Pharmaceuticals not only bought but also sold, collaborating with Sanofi on the development and commercialization of its Phase III product in the Chinese region.
- There were 8 transactions between domestic companies, which have essentially formed an integrated cooperation between innovative pharmaceutical companies with excellent R&D capabilities and those with domestic/global commercialization capabilities. Internationally, pharmaceutical companies with overseas sales channels, such as BeOne Medicines (BeiGene) and Kexing Phama, have partnered with CSPC Pharmaceutical Group and Kelun Pharmaceutical, respectively. In the domestic market, leading CSO company China Medical System Holdings (CMS) has partnered with Atom Therapeutics for anti-gout drugs. As outstanding innovative drugs enter the later stages of commercialization, cooperation between Chinese companies based on domestic and international markets will become increasingly frequent.-Fineline Info & Tech
Previous Recommendations