US-based biopharmaceutical company RAPT Therapeutics Inc. (NASDAQ: RAPT) has announced that it has obtained the ex-Greater China rights to Jiangxi Jemincare Group’s long-acting anti-IgE antibody, JYB1904, through a significant licensing agreement. The deal involves an upfront payment of USD 35 million and potential milestone payments of up to USD 672.5 million, in addition to sales royalties.
Global Rights Excluding Greater China for RPT904 Development
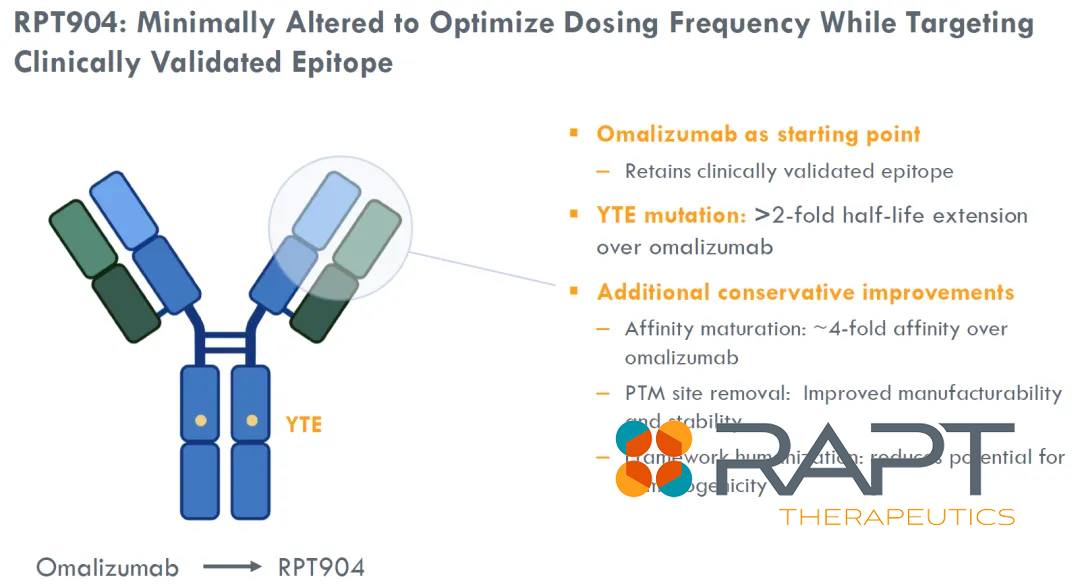
According to the agreement’s terms, RAPT Therapeutics has been granted worldwide rights to develop and commercialize RPT904, excluding mainland China, Hong Kong, Macau, and Taiwan. This Category 1 biologic product is a next-generation anti-IgE antibody with a demonstrated affinity four times higher than that of the established omalizumab. Furthermore, RPT904 boasts a half-life of 63 days, significantly surpassing the 27 days of omalizumab, which is currently the standard of care.
Phase II Clinical Trials and Future Prospects
RAPT Therapeutics’ newly acquired asset, RPT904, is currently undergoing Phase II clinical trials in China. The high affinity and extended half-life of RPT904 position it as a potentially transformative treatment in the management of IgE-mediated diseases, offering a more effective and longer-lasting alternative to existing therapies.-Fineline Info & Tech