-
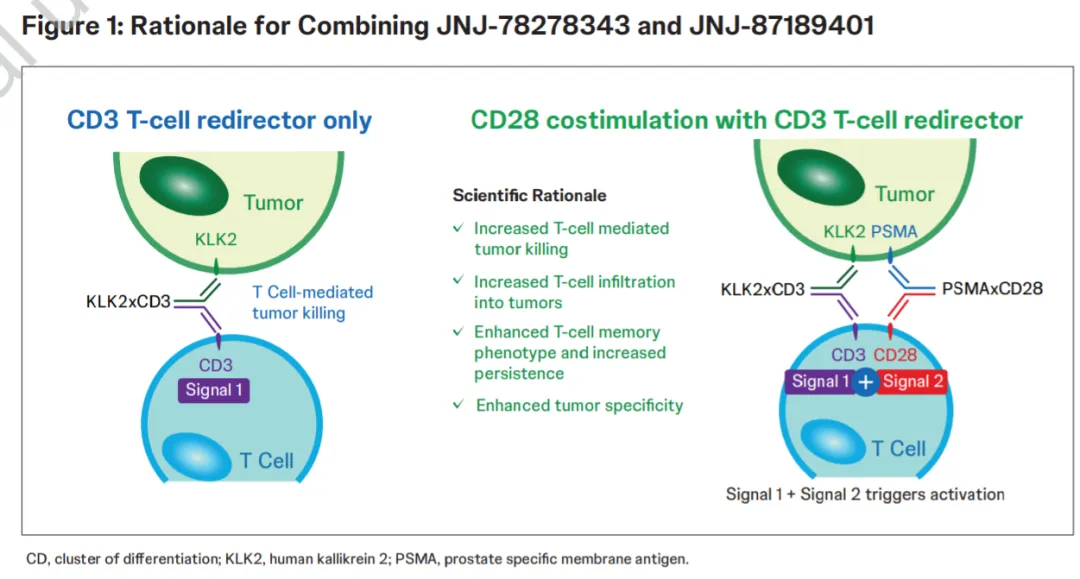
Johnson & Johnson’s KLK2-CD3 Bispecific Antibody JNJ-78278343 Advances in Prostate Cancer Clinical Trials
•
JNJ-78278343, a KLK2-CD3 bispecific antibody, is currently in Phase I clinical trials globally. According to data released by Johnson & Johnson (J&J, NYSE: JNJ), this drug targets kallikrein-related peptidase 2 (KLK2), an antigen highly expressed in prostate cancer, and binds to CD3 on T cells to activate them, inducing an…
-
Haisco Pharmaceutical’s BRAF Inhibitor HSK42360 Gains NMPA Approval for Clinical Trials
•
Haisco Pharmaceutical Group Co., Ltd (SHE: 002653), a leading pharmaceutical company based in China, has announced that it has secured another clinical trial approval from the National Medical Products Administration (NMPA) for its investigational drug HSK42360. This BRAF V600 inhibitor is now poised to be evaluated in clinical trials for…
-
Sperogenix Launches Early Access Program for DMD Therapy Agamgree in Hainan
•
Switzerland-based Santhera Pharmaceuticals (SWX: SANN) has announced that its Chinese partner Sperogenix Therapeutics has initiated an early access program (EAP) for the rare disease therapy Agamgree (vamorolone) in Hainan’s Bo’ao Lecheng Pilot Zone, with the first patients now receiving treatment for Duchenne muscular dystrophy (DMD). Sperogenix secured the rights to…
-
Ruijin Hospital Unveils Five New Mutations in Rare Adrenal Hyperplasia Study
•
In a groundbreaking study, Ruijin Hospital’s Endocrine Department researchers identified five novel mutations in 17 alpha-Hydroxylase/17,20 Lyase Deficiency (17OHD), a rare inherited adrenal hyperplasia. The research, spanning three years and encompassing eight patients from seven families, unveiled six mutations within the CYP17A1 gene, with five being previously unreported. The D487-F489del…
-
SFDA Embraces Transparency: Regular Press Briefings and Personnel Rotations Announced
•
China’s State Food and Drug Administration (SFDA) is gearing up for a transparency overhaul, with its head, Shao Mingli, unveiling a slate of reforms aimed at bolstering public and media scrutiny. Scheduled for the latter half of 2007, the agency will initiate a series of regular press conferences, marking a…
