JNJ-78278343, a KLK2-CD3 bispecific antibody, is currently in Phase I clinical trials globally. According to data released by Johnson & Johnson (J&J, NYSE: JNJ), this drug targets kallikrein-related peptidase 2 (KLK2), an antigen highly expressed in prostate cancer, and binds to CD3 on T cells to activate them, inducing an immune response against KLK2-positive tumor cells.
Clinical studies have indicated that KLK2 plays a pivotal role in the development of prostate cancer by promoting the transactivation of androgen receptors, accelerating the growth of prostate cancer cells. JNJ-78278343 is designed to bridge T cells and tumor cells, triggering T cell-mediated lysis of tumor cells, demonstrating potential immunomodulatory and antitumor effects.
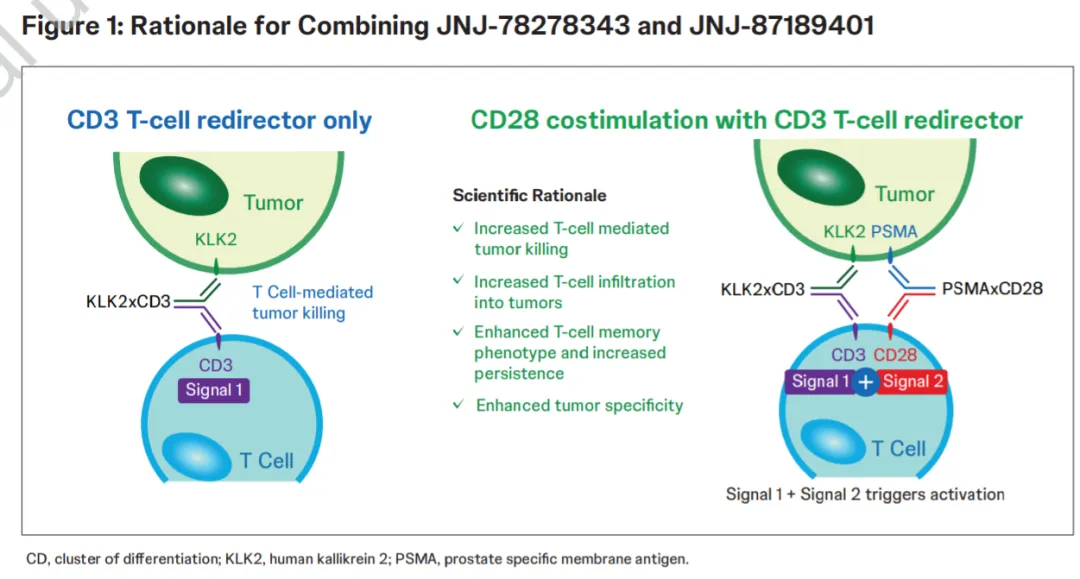
Information from ClinicalTrials.gov shows that Johnson & Johnson is conducting three Phase I clinical studies on JNJ-78278343, including monotherapy, combination therapy with the PSMA-CD28 bispecific antibody JNJ-87189401, and combination therapy with anti-PD-1 antibody cetrelimab, paclitaxel chemotherapy, or androgen receptor pathway inhibitors for metastatic castration-resistant prostate cancer.
At the 2024 European Society for Medical Oncology (ESMO) Congress, Johnson & Johnson presented the design of a Phase I clinical trial for the combination therapy of JNJ-78278343 and JNJ-87189401 in advanced prostate cancer. This combined therapy, by simultaneously activating the CD3 and CD28 pathways, may enhance T cell activation, leading to deeper and more durable clinical responses.
As a novel target for prostate cancer treatment, only a few targeted therapies against KLK2 have entered clinical studies globally. Johnson & Johnson has multiple innovative therapies in this field, including the KLK2-CD3 bispecific antibody, CAR-T cell therapy JNJ-75229414, and radiotherapeutic drug JNJ-69086420 (JNJ-6420), all of which are in Phase I clinical studies.
The progress in this field is not only of great significance to patients but also has a profound impact on advancing the medical community’s understanding and innovation in the treatment of prostate cancer. We look forward to the successful continuation of these studies and the early provision of more treatment options for prostate cancer patients.- Flcube.com