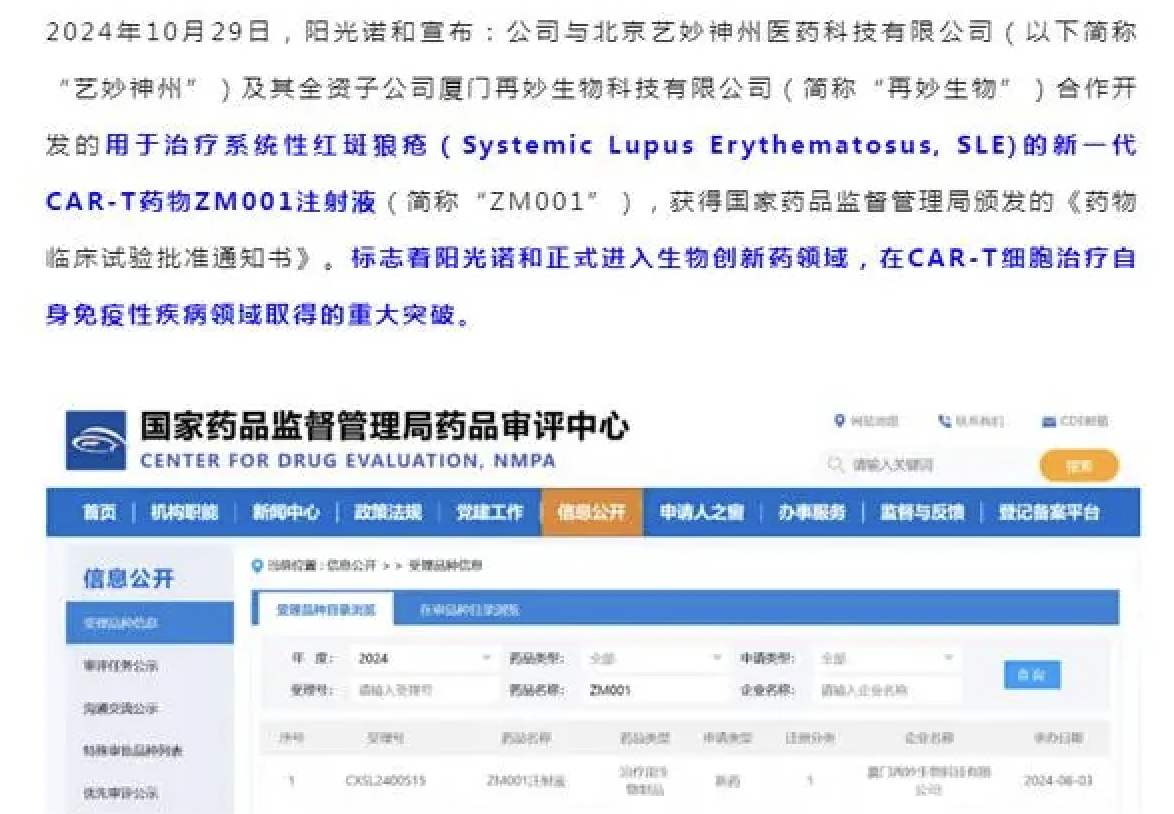
Beijing Sun-Novo Pharmaceutical Research Co., Ltd. (SHA: 688621) has secured approval from China’s National Medical Products Administration (NMPA) to initiate clinical trials for ZM001, a CD19-targeted chimeric antigen receptor (CAR)-T therapy, in the treatment of systemic lupus erythematosus (SLE). The therapy, co-developed with Beijing-based gene therapy specialist Immunochina Pharmaceuticals, is designed to selectively eliminate B cells in SLE patients, thereby inhibiting autoantibody production and reducing immune complex deposition in organs. This approach aims to facilitate the reconstitution of normal B cells, potentially leading to symptom alleviation and sustained therapeutic effects.
ZM001 has shown promising results in clinical studies, with rapid B cell clearance, enduring efficacy, and a notably low rate of severe adverse events, positioning it as a safe and potent CAR-T option for SLE patients. The collaboration between Sun-Novo and Immunochina to pursue ZM001 in SLE was established in June of this year.- Flcube.com