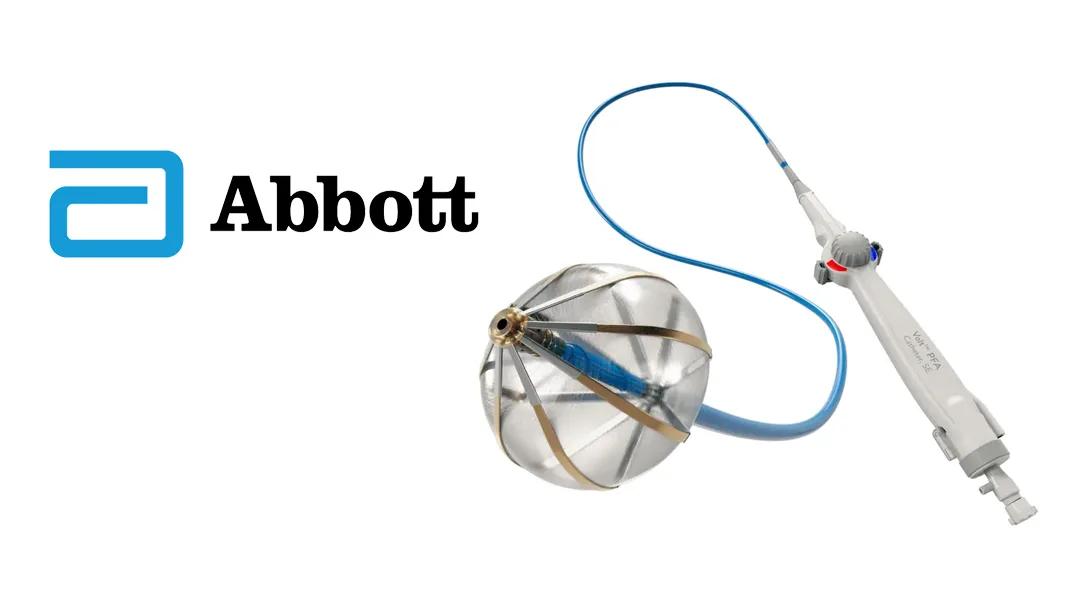
US-based Abbott Laboratories (NYSE: ABT) has announced the receipt of the CE mark in the European Union (EU) for its Volt PFA System, a novel therapy for patients with atrial fibrillation (AFib). This approval marks a significant advancement in treatment options for AFib, particularly for those with complex conditions.
Technology and Benefits
The Volt PFA system delivers high-energy electrical pulses to targeted areas of abnormal cardiac rhythms, effectively achieving ablation while reducing the risk of adjacent tissue damage. This is particularly beneficial for patients with complex diseases or anatomical structures.
Clinical Trial Success
The approval is supported by positive results from Abbott’s Volt CE Mark study, a global clinical trial conducted at centers in Europe and Australia. The Volt PFA System achieved pulmonary vein isolation (PVI) in 99.1% of veins during ablation procedures, using far fewer energy applications than other PFA systems on the market.-Fineline Info & Tech