Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd (HKG: 6990) has announced that the National Medical Products Administration (NMPA) in China has accepted for review another indication approval filing for its sacituzumab tirumotecan (SKB264/MK-2870). The new indication is for the treatment of locally advanced or metastatic EGFR mutant non-small cell lung cancer (NSCLC) that has progressed following treatment with epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI).
The filing is supported by positive results from the OptiTROP-Lung04 study, a multi-center, randomized, regulatory Phase III study. The study assessed the efficacy and safety of sacituzumab tirumotecan compared to pemetrexed combined with platinum therapy in patients with locally advanced or metastatic EGFR mutant NSCLC that has progressed after EGFR-TKI treatment. In a preset mid-term analysis, sacituzumab tirumotecan demonstrated significant statistical and clinical improvement in progression-free survival (PFS) as the primary endpoint, evaluated by the Blinded Independent Central Review (BICR), compared to pemetrexed combined with platinum-based drugs. The drug showed good safety, with no new safety signals identified.
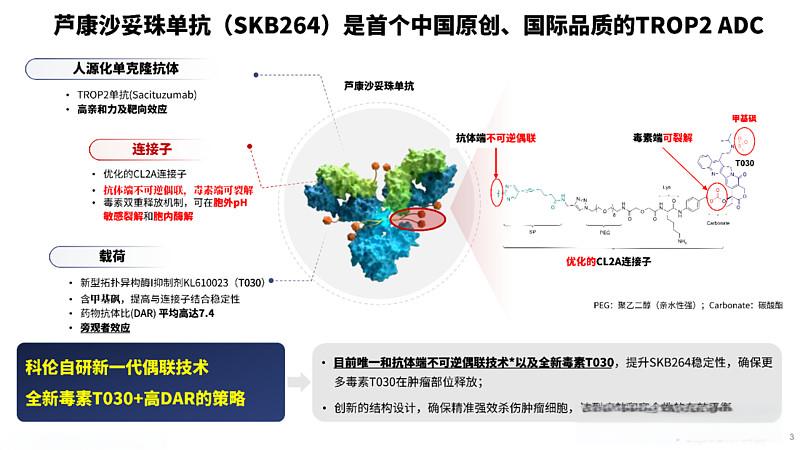
Sacituzumab tirumotecan (SKB264/MK-2870), a TROP2-targeted antibody-drug conjugate (ADC), is under priority review in China for the treatment of second-line and above triple-negative breast cancer (TNBC) and locally advanced or metastatic EGFR mutant NSCLC with EGFR-TKI and platinum-based chemotherapy failure. In May 2022, Merck, Sharp & Dohme (MSD) entered into a licensing deal for the drug for territories outside Greater China.- Flcube.com