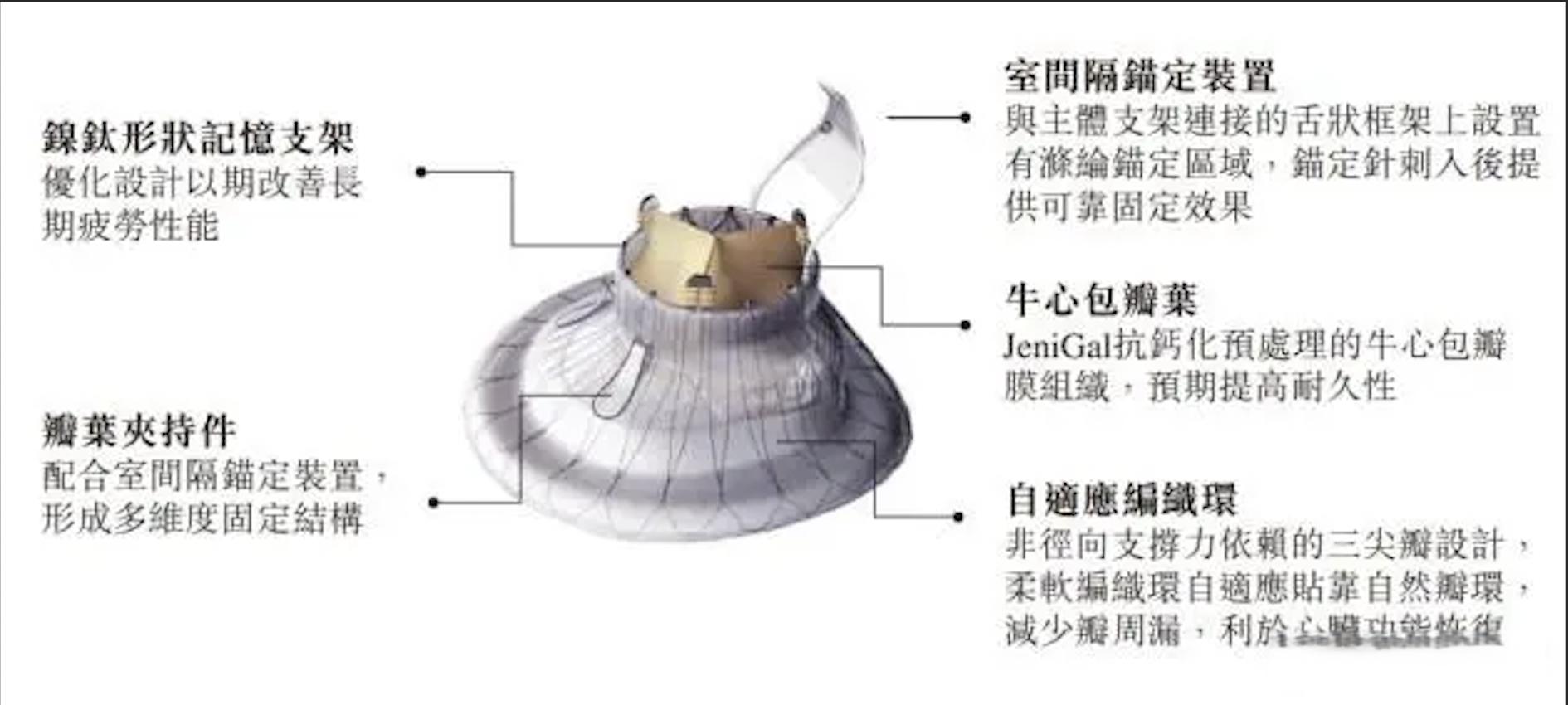
Ningbo-based structural heart disease device manufacturer Jenscare Scientific Co., Ltd (HKG: 9877) has announced the one-year follow-up results from the multi-center TRAVEL II study for its transjugular tricuspid valve replacement product, LuX-Valve Plus, at the Transcatheter Cardiovascular Therapeutics (TCT) 2024 annual meeting in Washington.
The TRAVEL II study, which assesses the long-term safety and efficacy of LuX-Valve Plus in patients with severe tricuspid regurgitation, enrolled 96 patients from 15 centers in China. The results indicated an improvement in tricuspid regurgitation grade in all patients, with 97.81% showing no or only minimal perivalvular leakage at 30 days, 97.62% showing no moderate or above regurgitation at 6 months, and 95.30% showing no moderate or above regurgitation at 1 year. In terms of New York Heart Association classification, 80% of patients improved from preoperative grade III/IV to grade I/II at 30 days, 92% at 6 months, and 85% at 1 year. Quality of life improvements were also noted, with an average increase of 15 points in the Kansas City Cardiomyopathy Questionnaire score at 30 days, 20 points at 6 months, and 21 points at 1 year. These results confirm LuX-Valve Plus’s excellent clinical performance in the medium and long term, maintaining a low incidence of safety events and continuous effectiveness improvement, leading to further heart function and quality of life enhancements for patients, and providing sustained clinical benefits.
Jenscare’s independently developed transcatheter tricuspid valve replacement system has been implanted in over 600 cases worldwide, with the longest follow-up record exceeding 6 years.- Flcube.com