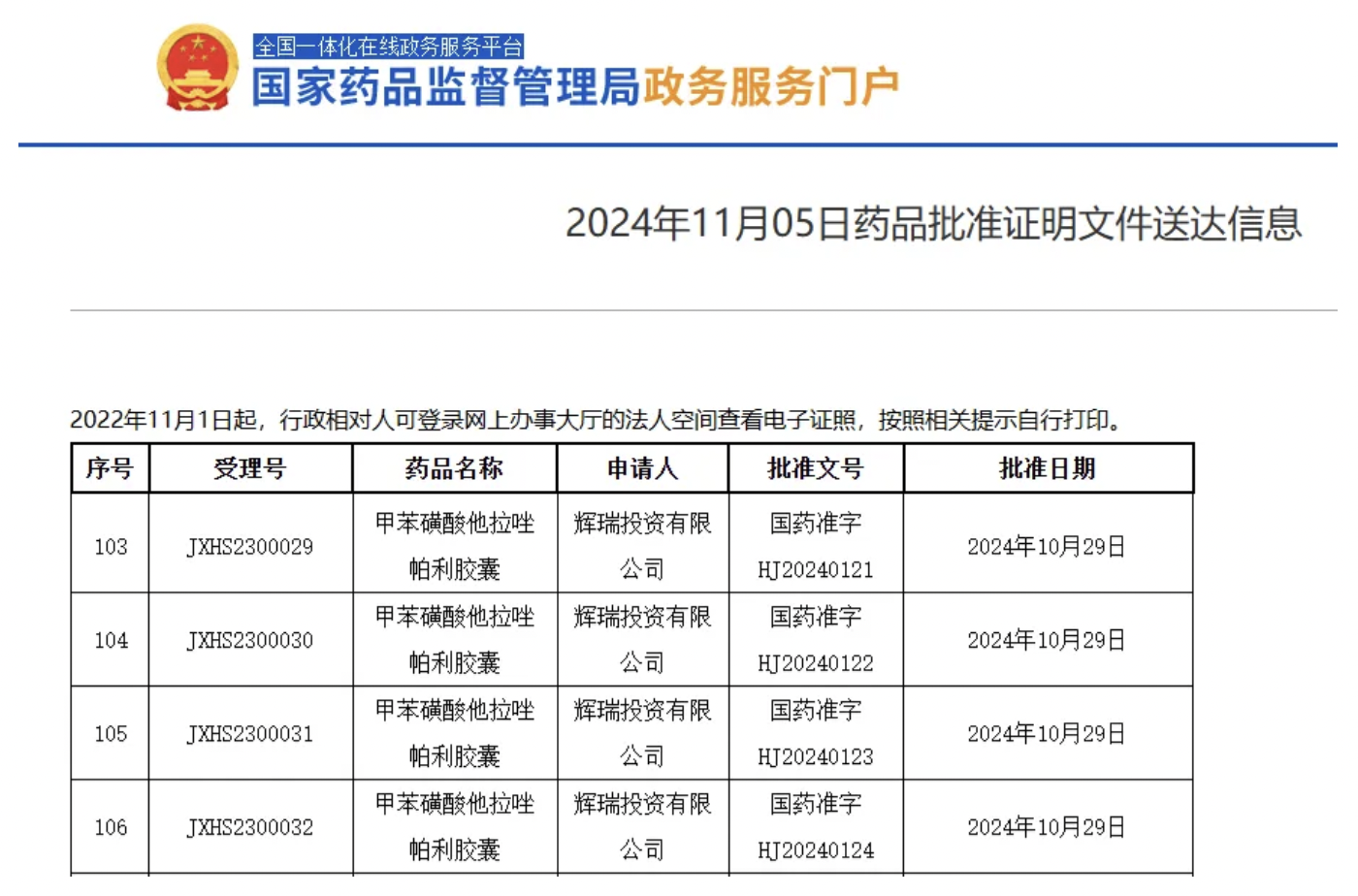
The National Medical Products Administration (NMPA) website has indicated that Pfizer’s (NYSE: PFE) marketing filing for Talzenna (talazoparib) has been approved, clearing the way for the poly ADP-ribose polymerase (PARP) inhibitor to treat metastatic castration-resistant prostate cancer (mCRPC) in China.
Talazoparib’s Global Approvals and Indications
Talazoparib received approval in the US in 2018 for the treatment of adult patients with BRCA mutant, human epidermal growth factor receptor 2 (HER2) negative, locally advanced or metastatic breast cancer. In June of the following year, it earned another indication for use in combination with enzalutamide to treat HRR mutant mCRPC patients.
Talazoparib’s Development History and Acquisition by Pfizer
Originally developed by US-based BioMarin, talazoparib was acquired by Medivation, a San Francisco-based cancer therapy developer, in 2015. Pfizer gained access to the drug in 2016 through its acquisition of Medivation for USD 14 billion.- Flcube.com