Johnson & Johnson MedTech (J&J, NYSE: JNJ) has announced that it has received marketing approval from the US Food and Drug Administration (FDA) for its Varipulse platform, marking a significant advancement in the treatment of drug refractory paroxysmal atrial fibrillation (AFib). The Varipulse platform is the company’s first pulsed field ablation (PFA) ablation catheter, offering a novel approach to managing this common cardiac arrhythmia.
The Varipulse Platform and Its Components
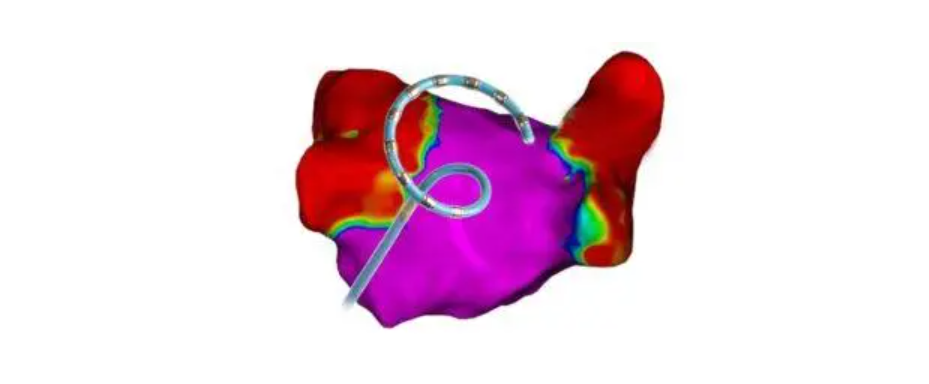
The Varipulse platform is a fully integrated system that includes the Varipulse catheter, Varipulse generator, and Carto 3 mapping system, along with Varipulse software. This comprehensive setup provides a seamless user experience by combining advanced ablation technology with precise mapping and visualization capabilities.
Integrating PFA Technology with Carto 3
As the first and only PFA technology integrated with the Carto 3 system, Varipulse offers intuitive and replicable workflows. The integration achieves real-time visualization and feedback mechanisms, enhancing the effectiveness and safety of the ablation procedure. This innovative platform is designed to improve patient outcomes by providing physicians with a more controlled and precise method for treating AFib.-Fineline Info & Tech