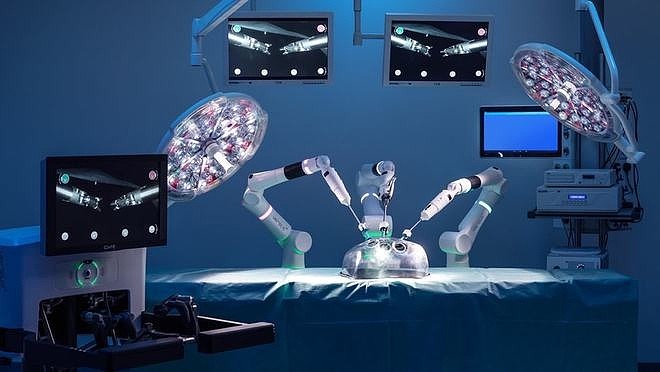
US-based Johnson & Johnson MedTech (J&J, NYSE: JNJ) has announced that it has received an investigational device exemption (IDE) from the US Food and Drug Administration (FDA), allowing its OTTAVA robotic surgical system to commence clinical trials at various sites across the United States. This approval marks a significant step in the development of advanced surgical technologies.
Innovative Features of the OTTAVA System
The OTTAVA System is designed to revolutionize the surgical experience by establishing a new standard for modern operating rooms (OR). It boasts a unique unified architecture and surgical instrumentation powered by Ethicon’s expertise, integrated within Johnson & Johnson MedTech’s digital ecosystem. This comprehensive system supports various surgical approaches, including robotic, laparoscopic, hybrid, and open surgery, enhancing the flexibility and efficiency of surgical procedures.
Clinical Trial Significance
The initiation of clinical trials for the OTTAVA System represents an important milestone for Johnson & Johnson MedTech as it seeks to validate the system’s performance and safety in real-world surgical settings. The outcomes of these trials will be critical in determining the system’s potential impact on surgical practices and patient outcomes.-Fineline Info & Tech