ImmuneOnco Biopharmaceuticals (Shanghai) Co., Ltd (HKG: 1541), a leading biopharmaceutical company specializing in immuno-oncology, has announced the commencement of a Phase II clinical study for its investigational drug, IMM27M, targeting estrogen receptor positive (ER+) advanced breast cancer following endocrine therapy failure or recurrence. This announcement coincides with the enrollment of the first patient in the study, marking a significant advancement in the development of novel treatments for breast cancer.
Positive Outcomes from Phase I Study
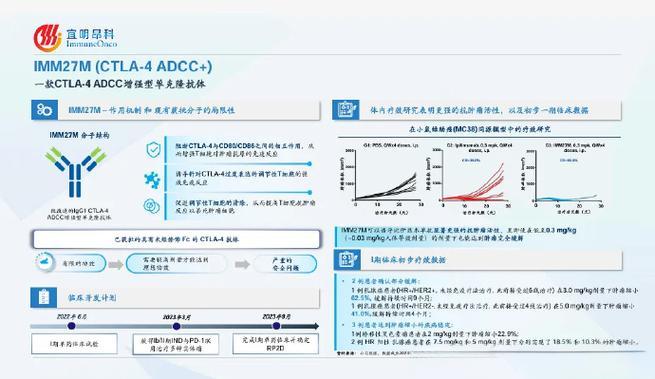
Building on the success of its Phase I dosage escalation study completed late last year, ImmuneOnco reported encouraging results among evaluable ER+ advanced or metastatic breast cancer patients. Notably, two patients achieved partial remission (PR), and four patients experienced stable disease (SD), resulting in an overall remission rate (ORR) of 25% and a disease control rate of 75%, alongside good tolerability. These findings underscore the potential of IMM27M as an effective treatment option for patients with advanced breast cancer.
Mechanism of IMM27M
IMM27M, an in-house developed ADCC (Antibody-Dependent Cellular Cytotoxicity) enhanced CTLA-4 antibody, is designed to comprehensively eliminate Treg (regulatory T) cells within the tumor microenvironment. This action enhances the anti-tumor response of T cells, offering a novel approach to treating advanced breast cancer by modulating the immune system to more effectively target and destroy cancer cells.-Fineline Info & Tech