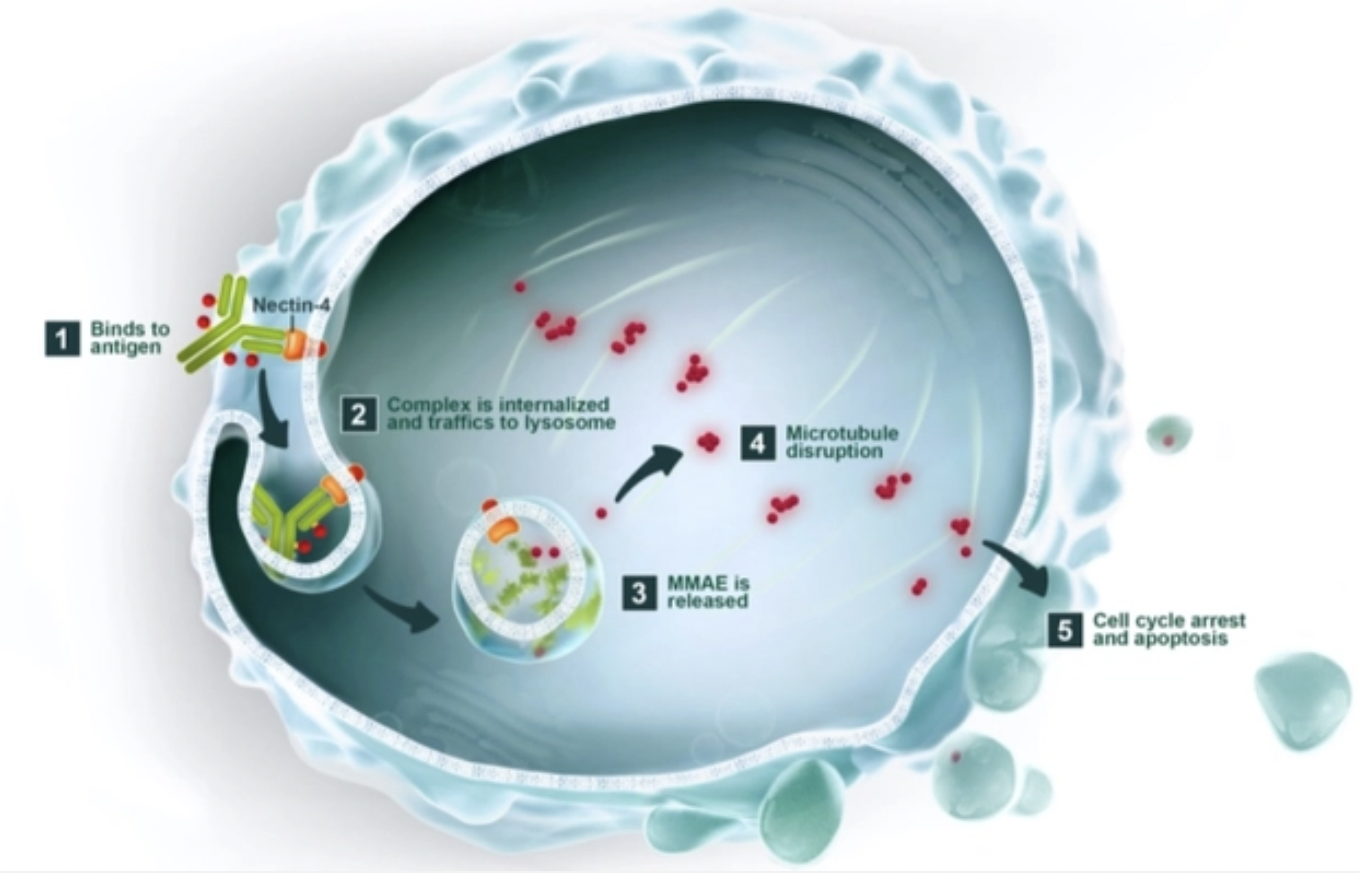
Mabwell (Shanghai) Bioscience Co., Ltd (SHA: 688062), a China-based biopharmaceutical company, has announced that it has received approval from the National Medical Products Administration (NMPA) to initiate clinical studies for its antibody drug conjugate (ADC), 9MW2821. This drug targets Nectin-4 and will be evaluated in combination with programmed-death 1 (PD-1) inhibitors for perioperative urothelial carcinoma, as well as with other anti-tumor drugs for advanced solid tumors.
Clinical Study Design and Significance
The clinical studies for 9MW2821 will explore the efficacy and safety of the drug in combination therapies, providing a potential new treatment option for patients with urothelial carcinoma and advanced solid tumors. This approach aims to leverage the synergistic effects of combining 9MW2821 with PD-1 inhibitors and other anti-tumor agents, enhancing the treatment outcomes for these cancers.
9MW2821: A Milestone in Chinese Biotechnology
9MW2821 marks a significant milestone as the first domestically developed Nectin-4 targeted ADC in China to enter clinical trials. The drug is already the subject of multiple clinical studies for esophageal cancer and urothelial carcinoma (UC), indicating its potential in addressing a range of cancer types. The initiation of these clinical studies represents a step forward in the development of innovative cancer therapies in China and globally.-Fineline Info & Tech