Sino Biopharmaceutical Ltd (HKG: 1177), a leading China-based pharmaceutical company, has announced that it has received clearance from the US Food and Drug Administration (FDA) to initiate a Phase I clinical study for its TQB3002. This is an in-house developed fourth-generation epidermal growth factor receptor (EGFR) inhibitor, marking a significant step in the advancement of targeted therapies for non-small cell lung cancer.
EGFR Inhibition and TQB3002’s Potential
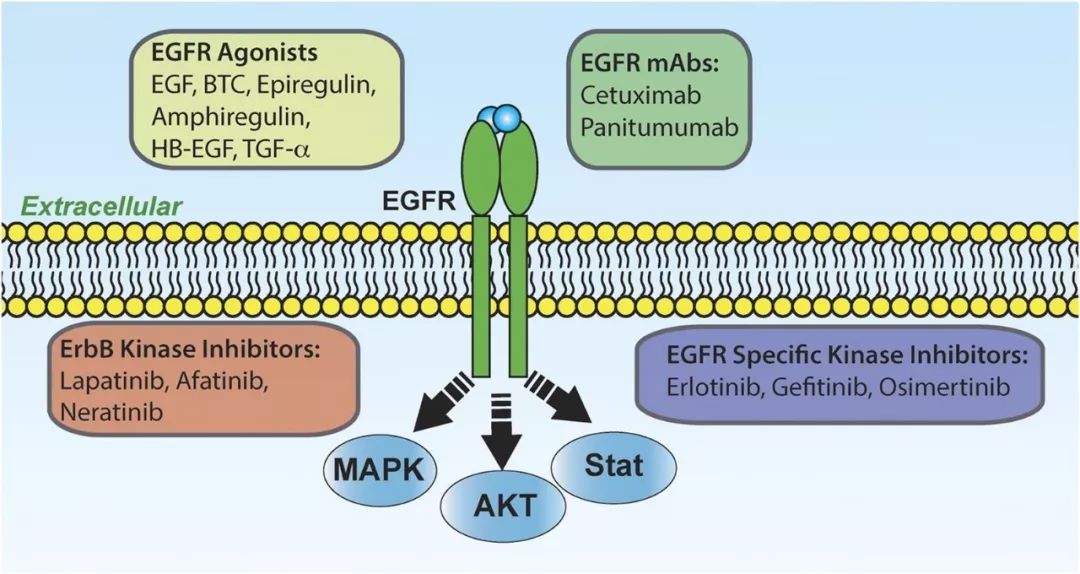
Epidermal growth factor receptor (EGFR) is one of the genes with the highest mutation frequency and a prominent driver in non-small cell lung cancer. The mutation rate ranges from 40% to 50% in East Asian populations and 10% to 20% in Western populations. TQB3002 competitively binds to the ATP site of the intracellular tyrosine kinase binding domain, inhibiting the activity of related tyrosine kinases and intracellular phosphorylation processes. This inhibition of downstream EGFR signaling ultimately leads to tumor cell death. TQB3002 is designed to address drug resistance issues observed in previous generations of EGFR inhibitors.
Preclinical Success and Clinical Trial Initiation
Preclinical studies have demonstrated that TQB3002 can inhibit kinase activity in single EGFR mutations (EGFRd746-750 and EGFRL858R) and dual EGFR mutations (EGFRd746-750/T790M and EGFRL858R/T790M). The drug has shown strong inhibitory activity in single, dual-, and triple- EGFR mutant cell lines. In in vivo models, TQB3002 has inhibited tumor growth in a dose-dependent manner and has exhibited good tolerability. These promising preclinical results have paved the way for the initiation of Phase I clinical trials, which will further evaluate the safety, tolerability, and efficacy of TQB3002 in human subjects.-Fineline Info & Tech