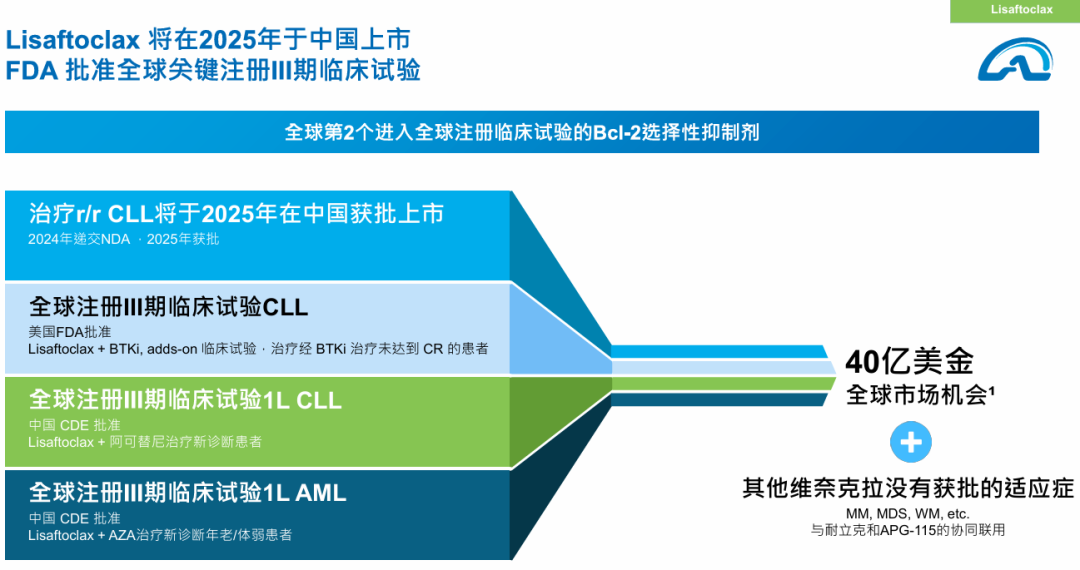
Ascentage Pharma (HKG: 6855) has announced that its proprietary oral Bcl-2 selective inhibitor, Lisaftoclax (APG-2575), has submitted its market application, which has been officially accepted by regulatory authorities. The formulation is being produced in collaboration with Xuantai Pharmaceutical. This innovative treatment targets patients suffering from refractory or relapsed chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL).
A Promising Alternative in Blood Cancer Treatment
Lisaftoclax stands out as the world’s second Bcl-2 inhibitor to reach this stage, offering improved safety profiles and dose escalation timelines compared to its predecessor, Venetoclax. The drug demonstrates significant therapeutic potential across various hematologic malignancies and solid tumors. Remarkably, the objective response rates (ORR) for first-line and relapsed/refractory (r/r) CLL/SLL patients treated in combination with BTK inhibitors reached impressive levels of 100% and 98%, respectively. As of now, Ascentage Pharma has an estimated market capitalization of HKD 13.3 billion on the Hong Kong Stock Exchange.
Mechanism of Action and Orphan Drug Designation
Lisaftoclax functions through the selective inhibition of the Bcl-2 protein, reactivating the normal apoptosis process in cancer cells, thereby targeting tumor growth. The compound has previously received five Orphan Drug Designations from the U.S. FDA, which include indications for Waldenström’s macroglobulinemia (WM), chronic lymphocytic leukemia (CLL), multiple myeloma (MM), acute myeloid leukemia (AML), and follicular lymphoma (FL).-Fineline Info & Tech