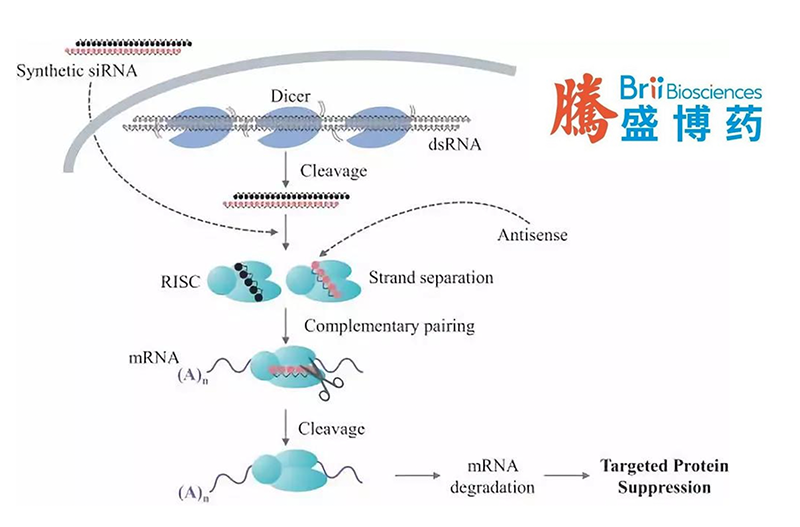
China-based Brii Biosciences Ltd (HKG: 2137) has published the latest data from its ongoing Phase II ENSURE study for elebsiran (BRII-835, VIR-2218), an investigational small interfering ribonucleic acid (siRNA) therapy for chronic hepatitis B virus (HBV) infection.
Phase II ENSURE Study Design and Initial Findings
The active-controlled and randomized Phase II study aims to evaluate the efficacy of elebsiran in combination with pegylated interferon alpha (PEG-IFNα) in participants with chronic HBV infection and baseline hepatitis B surface antigen (HBsAg) levels of 100-3,000 IU/mL. The study excludes patients with low baseline HBsAg levels (<100 IU/mL) to focus on the potential curative benefits in a broader patient population. The 48-week end-of-treatment (EOT) data from the ENSURE study showed that 26.3% (5/19) of participants receiving 200 mg or 33.3% (6/18) receiving 100 mg elebsiran in combination with PEG-IFNα achieved HBsAg seroclearance at EOT, compared to 5.6% (1/18) receiving PEG-IFNα alone.
Elebsiran’s Mechanism of Action and Development Rights
Elebsiran is an investigational subcutaneously administered HBV-targeting siRNA designed to degrade hepatitis B virus RNA transcripts and limit the production of hepatitis B surface antigen. Brii Bio entered into a licensing agreement with Vir Biotechnology, Inc. (Nasdaq: VIR) in 2020, securing exclusive rights to develop and commercialize elebsiran in Greater China. Elebsiran is also being tested in combination with monoclonal antibody BRII-877 (tobevibart) or immunotherapy BRII-179 for the treatment of HBV infection.-Fineline Info & Tech