Germany-based Bayer AG (ETR: BAYN) has announced a significant collaboration and license agreement with US-based Cytokinetics, Incorporated (NASDAQ: CYTK), acquiring exclusive development and commercialization rights to aficamten in Japan. The agreement is subject to certain reserved development rights of Cytokinetics.
Phase III Study and Global Trial Expansion
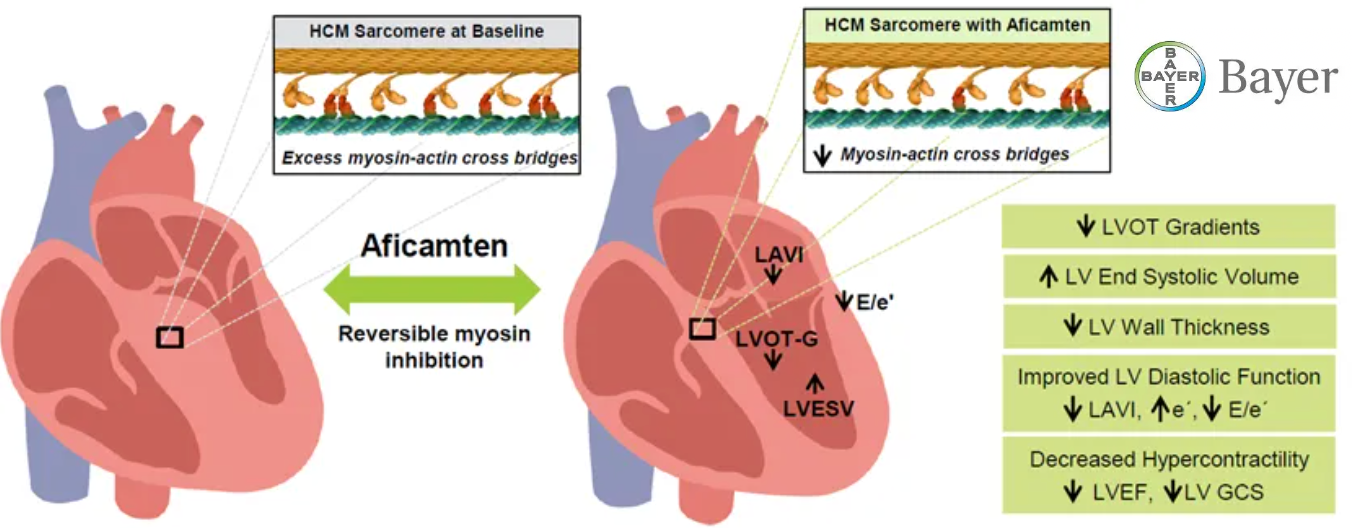
Under the terms of the deal, Bayer is expected to conduct a Phase III study for aficamten, a cardiac myosin inhibitor intended to treat obstructive and non-obstructive hypertrophic cardiomyopathy (HCM), specifically in Japanese patients with HCM. Concurrently, Cytokinetics will expand ACACIA-HCM, the ongoing global Phase III clinical trial of aficamten in patients with non-obstructive HCM, into Japan. This expansion aims to support the potential market filing for aficamten in Japan for Bayer and CEDAR-HCM, the study focused on a pediatric population with obstructive HCM.
Financial Terms and Milestones
Cytokinetics will receive an upfront payment of EUR 50 million (USD 52.91 million) and is eligible to receive up to an additional EUR 90 million (USD 95.24 million) upon the achievement of milestones through commercial launch, including EUR 20 million (USD 21.17 million) which are near-term. Furthermore, Cytokinetics stands to receive up to EUR 490 million (USD 518.5 million) in commercial milestone payments upon the achievement by Bayer of certain sales milestones, along with tiered royalties on net sales of aficamten in Japan.
Aficamten’s Licensing History and Global Reach
Aficamten, originated by Cytokinetics, was previously the subject of a licensing deal with Ji Xing Pharmaceuticals in July 2020, granting the Chinese firm exclusive development and commercialization rights to the drug in Greater China. Regulatory decisions for aficamten in China are currently pending.-Fineline Info & Tech