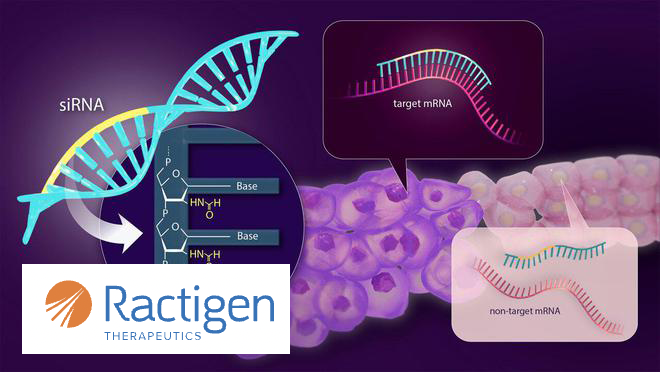
China-based small activating RNA (saRNA) drug developer, Ractigen Therapeutics, has announced that it has received Orphan Drug Designation (ODD) from the US Food and Drug Administration (FDA) for its drug candidate RAG-21. This FUS gene-targeted siRNA therapy is indicated for the treatment of amyotrophic lateral sclerosis (ALS), an incurable and devastating neurodegenerative disease.
Understanding ALS and the Impact of FUS Gene Mutations
ALS is a neurodegenerative disease that selectively damages motor neurons in the cortex and spinal cord, leading to respiratory failure within 2-5 years of diagnosis for most patients. Mutations in the FUS gene are associated with an aggressive form of ALS, characterized by early onset and rapid progression. These mutations result in toxic protein accumulation, mis-localization, and the formation of abnormal inclusions in neurons, ultimately leading to motor neuron degeneration.
RAG-21’s Mechanism of Action and Pre-Clinical Promise
RAG-21 utilizes RNA interference to selectively reduce FUS mRNA transcripts, preventing the production of toxic FUS proteins and addressing the underlying pathology of FUS-ALS. Pre-clinical studies have demonstrated that RAG-21 has a significant knockdown effect on FUS mRNA and shows good safety, holding the potential to bring prognostic improvement to FUS-ALS patients who currently lack disease-modifying therapies.-Fineline Info & Tech