China-based CSPC Pharmaceutical Group Co., Ltd (HKG: 1093) has announced that it has received Fast Track designation from the US Food and Drug Administration (FDA) for its drug candidate CRB-701 (SYS6002), which is being developed for the treatment of recurrent/refractory metastatic cervical cancer.
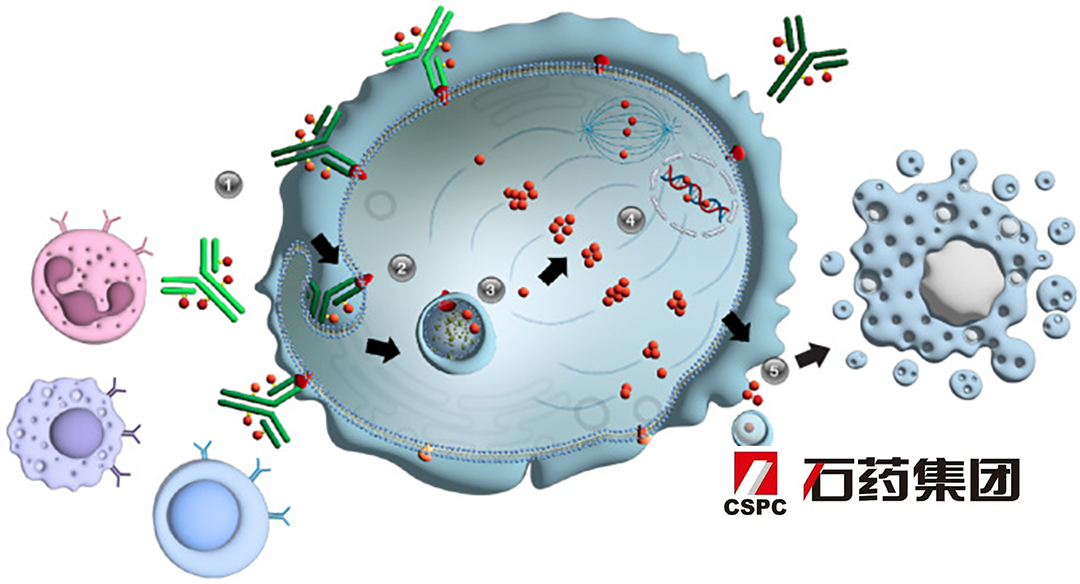
CRB-701 (SYS6002): An Innovative ADC Targeting Nectin-4
SYS6002 is an antibody drug conjugate (ADC) that utilizes CSPC’s proprietary enzyme catalyzed site-specific antibody conjugate technology. It targets Nectin-4, a protein that can promote tumor proliferation, angiogenesis, lymphangiogenesis, and lymphatic metastasis, and is selectively highly expressed in various cancers. This targeted approach has the potential to improve outcomes for patients with cervical cancer.
Global Licensing Deal with Corbus Pharmaceuticals
In February 2023, CSPC entered into a significant licensing agreement with US firm Corbus Pharmaceuticals (FRA: 3371), granting the company development and commercialization rights to SYS6002 in the US, European Union (EU), the UK, and other territories. The deal, valued at USD 700 million, underscores the global potential of CRB-701 and the strategic partnership to bring this innovative treatment to market.-Fineline Info & Tech