US major Johnson & Johnson MedTechhas announced receiving premarket approval (PMA) from the US Food and Drug Administration (FDA) for its Impella 5.5 with SmartAssist and Impella CP with SmartAssist heart pumps. This approval expands their indications to include specific pediatric patients with symptomatic acute decompensated heart failure (ADHF) and cardiogenic shock.
Impella: A Pioneering Artificial Heart Assistive Device
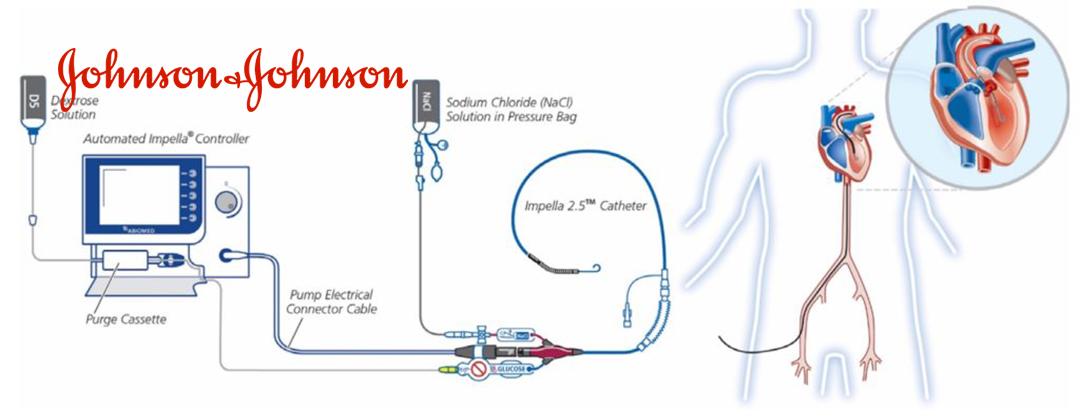
Impella, an artificial heart assistive device developed by Abiomed Inc., was acquired by Johnson & Johnson (J&J, NYSE: JNJ) for a substantial USD 16.6 billion in November 2022. This interventional artificial heart product is the only one with an FDA nod and is approved in the US, European Union (EU), and Japan for a range of cardiovascular applications. These include use in cardiogenic shock, left ventricular unloading in conjunction with ECMO, high-risk PCI treatment, ventricular tachycardia ablation, and right ventricular failure.
Expanding Access to Pediatric Cardiac Care
The latest FDA approval marks a significant expansion of Impella’s use to younger patient populations, offering a potentially life-saving option for pediatric patients suffering from acute decompensated heart failure and cardiogenic shock. This development underscores Johnson & Johnson’s commitment to advancing pediatric medical technology and addressing the critical needs of this vulnerable patient group.-Fineline Info & Tech