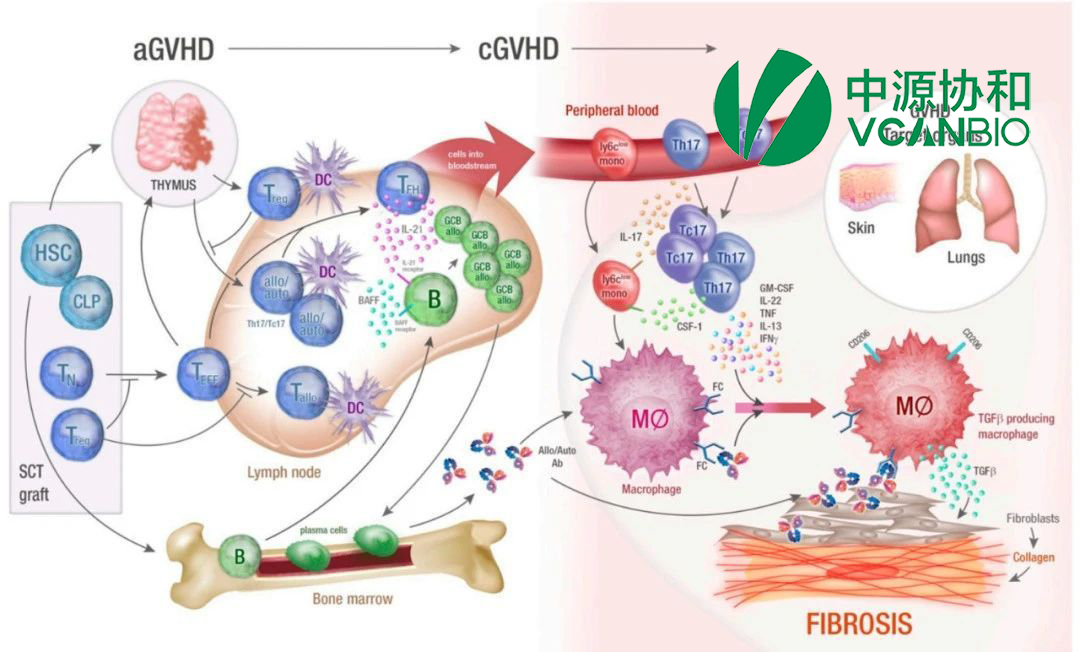
China-based Vcanbio Cell & Gene Engineering Corp., Ltd (SHA: 600645) has announced significant progress for its in-house developed VUM02 for injection, receiving approval from the National Medical Products Administration (NMPA) in China to study the treatment of decompensated cirrhosis and obtaining Orphan Drug Designation (ODD) from the Food and Drug Administration (FDA) in the United States for acute graft-versus-host disease (aGvHD).
VUM02: A Category 1 Therapeutic Biologic Product
VUM02 is a Category 1 therapeutic biologic product, essentially consisting of human umbilical cord-derived mesenchymal stem cells. These cells are stored under liquid nitrogen conditions, which allows for a long shelf life. Previous studies have demonstrated VUM02’s good safety and tolerability, along with a trend towards efficacy.
FDA’s ODD and Its Implications for VUM02
The FDA had previously granted VUM02 ODD status for the treatment of idiopathic pulmonary fibrosis last year. The latest ODD for aGvHD further highlights the potential of VUM02 as a treatment for rare and severe diseases, which may qualify it for special incentives to support its development and market entry.-Fineline Info & Tech