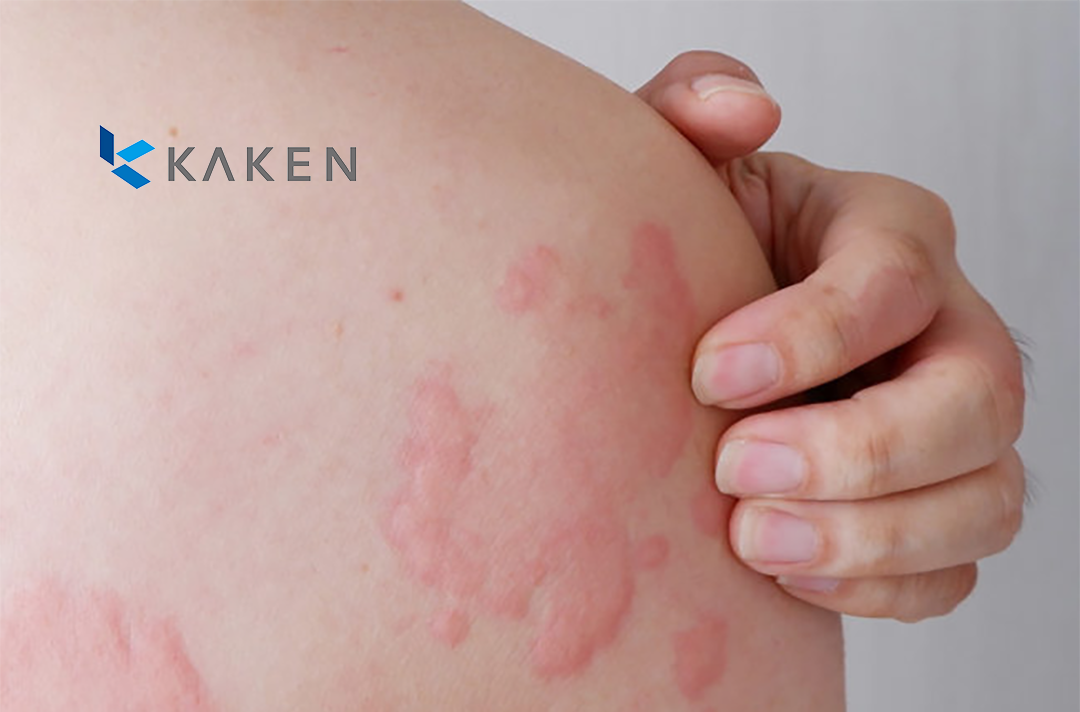
US giant Johnson & Johnson (J&J, NYSE: JNJ) has announced an exclusive licensing agreement with Japan-based Kaken Pharmaceutical Co., Ltd. This agreement grants Johnson & Johnson global development, manufacturing, and commercialization rights for a STAT6 program aimed at treating autoimmune and allergic diseases, including atopic dermatitis (AD). Under the terms of the deal, Kaken will retain commercialization rights in Japan, where Johnson & Johnson will have the option to enter into a co-promotion agreement.
KP-723: A Promising Candidate for Autoimmune and Allergic Diseases
The STAT6 program centers around KP-723, a drug that modulates the signal transducer and activator of transcription 6 (STAT6) to intervene in autoimmune and allergic diseases. A Phase I study for KP-723 in atopic dermatitis is slated to begin next year. This innovative therapy has the potential to treat asthma and other Th2-mediated diseases, complementing J&J’s existing portfolio of molecules targeting pathways relevant to atopic dermatitis.
Investment Opportunities and Strategic Collaboration
In addition to the licensing agreement, Kaken Pharmaceutical is eligible to receive an equity investment from Johnson & Johnson Innovation – JJDC, Inc., the venture capital arm of Johnson & Johnson. This strategic collaboration not only enhances J&J’s capabilities in the field of autoimmune and allergic disease treatments but also reinforces its commitment to advancing innovative therapies that address unmet medical needs.-Fineline Info & Tech