China-based Simcere Pharmaceutical Group Limited’s (HKG: 2096) subsidiary, Simcere Zaiming, has announced that it has received clinical trial approval from the National Medical Products Administration (NMPA) for its antibody drug conjugate (ADC) SIM0505, which targets CDH6 in advanced solid tumors. Last month, the drug also received clearance for clinical trials in the US.
Drug Development and Targeting Mechanism
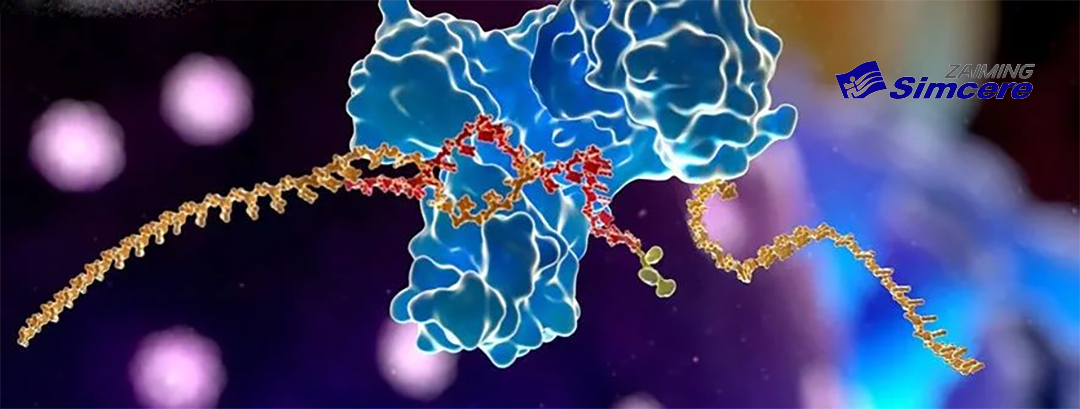
SIM0505 is designed for multi-center clinical studies globally. It combines the tumor-specific targeting of the CDH6 antibody molecule with the high cytotoxicity of the camptothecin toxin molecule. This combination offers therapeutic potential for malignant tumors such as ovarian and renal cancer.
Significance of CDH6 Targeting
CDH6, also known as cadherin 6 or K-cadherin, is a single transmembrane protein involved in intercellular adhesion, organ development, and epithelial mesenchymal transition. It is significantly overexpressed in advanced ovarian and renal cancers, making it an important target for the treatment of advanced tumors. The approval for clinical trials marks a significant step in exploring SIM0505’s potential in addressing these challenging conditions.-Fineline Info & Tech