China-based Keymed Biosciences Inc. (HKG: 2162) and InnoCare Pharma (HKG: 9969, SHA: 688428) have jointly announced a licensing agreement with Prolium, a US-based biotech backed by RTW Investments. Under the terms of the agreement, Prolium will obtain development, regulatory filing, manufacturing, and commercialization rights to ICP-B02 (CM355), a CD20xCD3 bispecific antibody (BsAb), in the non-oncology field globally and in the oncology field outside Asia.
ICP-B02: Mechanism and Development
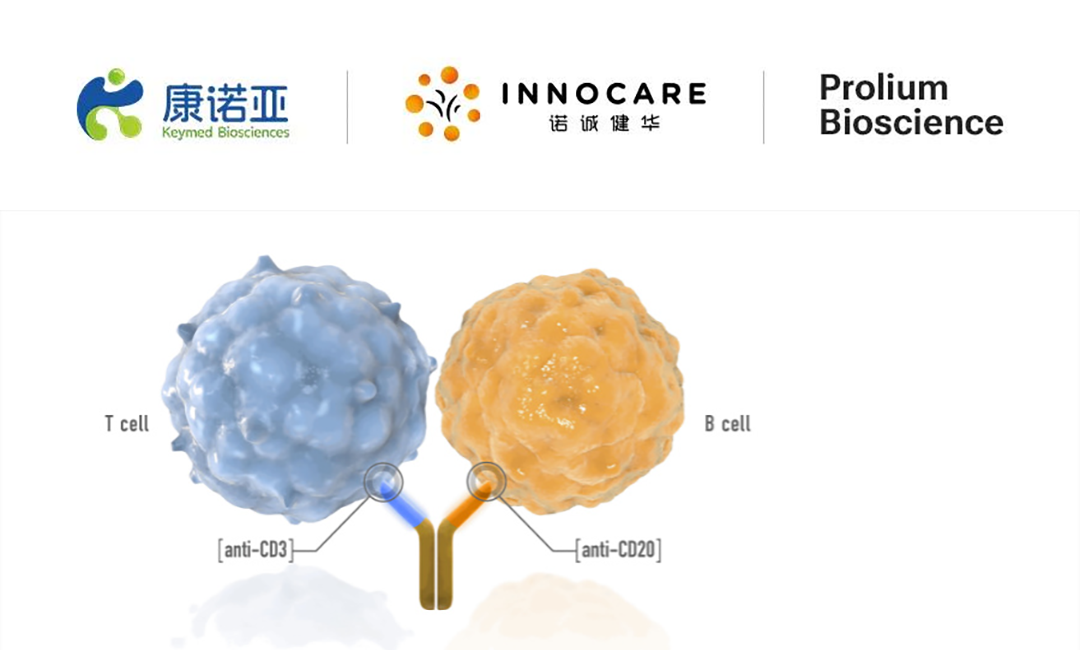
ICP-B02, a CD20xCD3 bispecific antibody jointly developed by InnoCare and Keymed, is designed to specifically bind to CD20-positive target cells and CD3-positive T cells. By recruiting immune T cells to the periphery of target cells, the antibody activates T cells and induces T cell-mediated tumor cell killing (TDCC) to eliminate target cells. This innovative mechanism positions ICP-B02 as a promising therapeutic candidate for both oncology and non-oncology indications.
Financial Terms and Future Collaboration
Under the agreement, InnoCare and Keymed will receive up to USD 520 million in total, including upfront and near-term payments, as well as clinical, regulatory, and commercial milestone payments. Additionally, the companies will hold a minority equity stake in Prolium and are eligible to receive tiered royalties on future net sales of any product resulting from the collaboration. This partnership underscores the potential of ICP-B02 in expanding therapeutic options globally.-Fineline Info & Tech