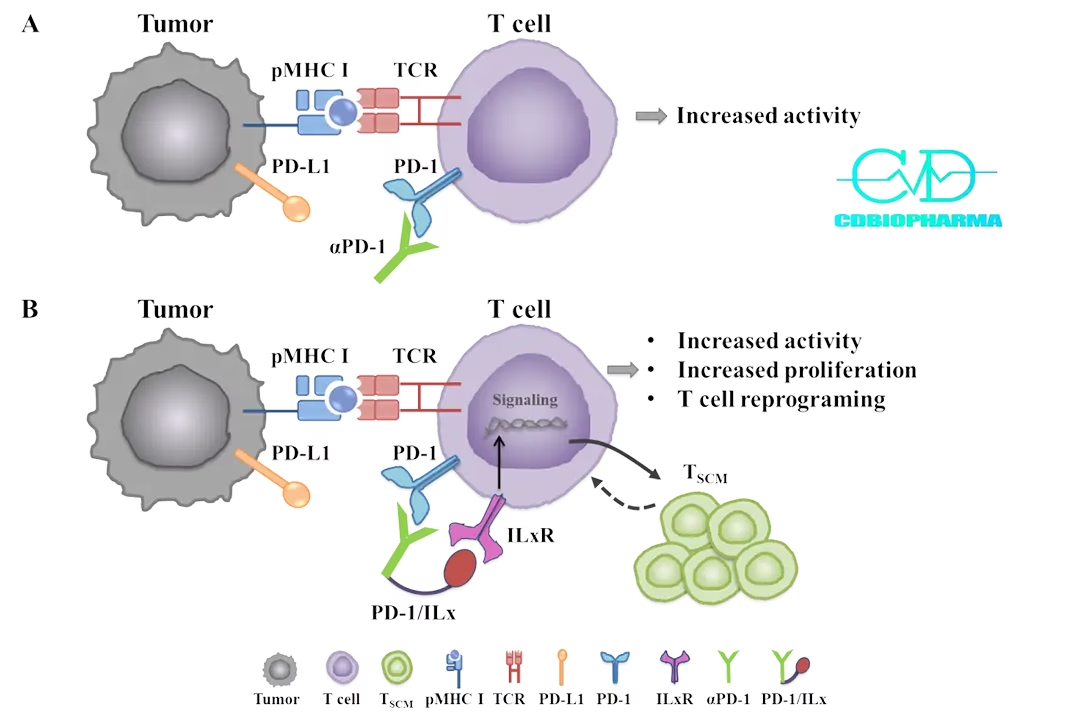
Suzhou-based tumor immunotherapy specialist CD Biopharma has announced the first subject dosing of a Phase I study for its novel anti-tumor drug CD-001 in patients with advanced solid tumors. The patient is reported to be stable with all indicators being normal. This milestone marks the beginning of clinical evaluation for CD-001, an innovative therapy targeting CD8+ T cells.
CD-001: Mechanism and Development
CD-001 is an IL-21 fusion protein designed to enhance the efficacy of cancer treatments, particularly addressing the limitations of PD-1 inhibitors in clinical settings. Preclinical studies have demonstrated significant anti-tumor therapeutic effects in various mouse tumor models, with CD-001 showing robust safety and pharmacokinetic profiles in non-human primate toxicology studies. The drug is currently under clinical review in China for blood cancer and has received clearance for trials in advanced solid tumors in both China and the US.
Future Outlook
With the initiation of the Phase I study, CD Biopharma is poised to further explore the potential of CD-001 in addressing unmet needs in cancer treatment. The drug’s unique mechanism of action and promising preclinical data position it as a potential breakthrough in the field of tumor immunotherapy.-Fineline Info & Tech