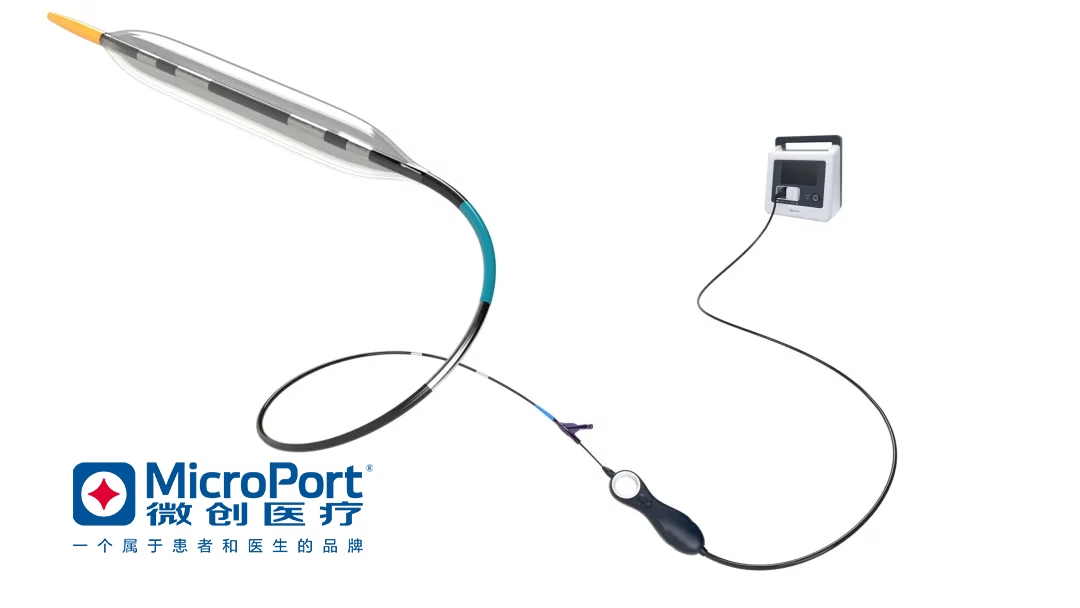
Shanghai-based medical device giant MicroPort Scientific Corp. (HKG: 0853) has announced receiving marketing approval from the National Medical Products Administration (NMPA) for its TomaHawk intravascular shock wave therapy device, disposable coronary intravascular shock wave catheter (TomaHawk coronary intravascular shock wave catheter system). This approval marks a significant milestone in the commercialization of this innovative medical device.
Device Profile and Technology
The TomaHawk coronary intravascular shock wave catheter system, developed utilizing innovative intravascular lithotripsy (IVL) technology, is designed to reduce operational complexity and the risk of complications. It aims to improve the safety of coronary intervention surgery while reducing operational difficulty and shortening the learning curve for operators. The system promotes the popularization of pre-treatment for moderate to severe calcified lesions in clinical applications, thereby benefiting more patients. The system offers 18 specification options, providing flexibility to meet diverse clinical needs.
Future Prospects and Strategic Implications
The marketing approval from the NMPA positions MicroPort Scientific Corp. to further expand its presence in the cardiovascular device market. By leveraging its innovative technology and extensive product portfolio, MicroPort aims to address significant unmet medical needs and contribute to the advancement of cardiovascular care. This approval underscores MicroPort’s commitment to innovation and improving patient outcomes through advanced medical devices.-Fineline Info & Tech