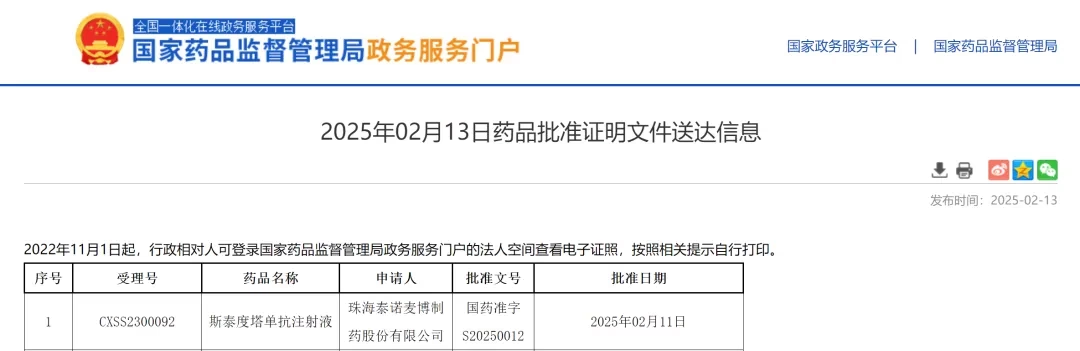
China-based Trinomab Biotech Co., Ltd has received market approval for its antitetanus toxin monoclonal antibody (mAb) injection TNM002 from the National Medical Products Administration (NMPA).
Product Details
TNM002 is a Category 1 product, an iterated novel tetanus shot used for emergency prevention of tetanus in adults. It delivers emergency protection through intramuscular injection with a quick effect.
Technology and Advantages
Based on Trinomab’s HitmAb technology platform, TNM002 differentiates itself from traditional tetanus antitoxin (TAT) derived from horse blood products and human tetanus immunoglobulin (HTIG) derived from human plasma. Studies have shown that 95.4% of patients vaccinated with TNM002 achieved protection 12 hours after administration, significantly higher than the 53.2% achieved with HTIG.-Fineline Info & Tech