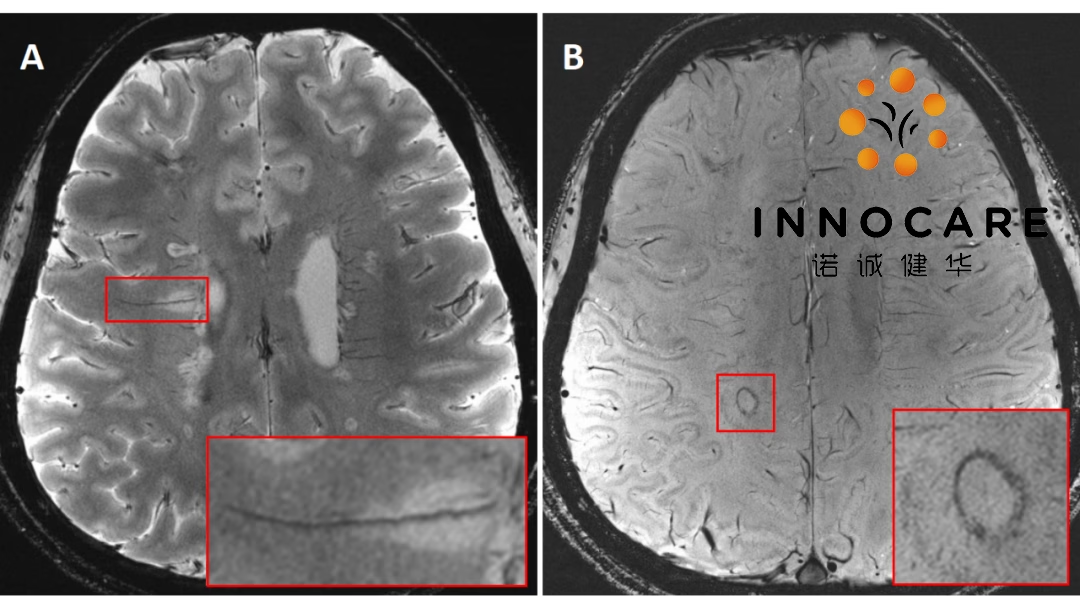
Beijing-based InnoCare Pharma (HKG: 9969, SHA: 688428) announced the presentation of Phase II study results for its Bruton’s tyrosine kinase (BTK) inhibitor orelabrutinib in relapsing-remitting multiple sclerosis (RRMS) at the 10th annual Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum.
Study Highlights
The data demonstrated significant efficacy of orelabrutinib in RRMS patients. The 80 mg once daily (QD) dose showed the best efficacy and safety profile, leading to its selection for Phase III progressive MS studies.
Key Results
- At Week 12, all treatment groups showed statistically significant reductions in new Gd+ T1 lesions and new/enlarging T2 lesions compared to placebo (p < 0.05).
- The 80 mg QD group achieved the highest reductions: 90.4% at Week 12 and 92.3% at Week 24 compared to placebo.
- New lesion control was observed as early as 4 weeks and sustained through 24 weeks.
Conclusion
The Phase II study supports the potential of orelabrutinib as a therapeutic option for RRMS, with the 80 mg QD dose showing superior efficacy and safety. Future Phase III trials will further evaluate its impact on progressive MS.-Fineline Info & Tech